Press Releases
• PenKid surpasses serum creatinine on Day 1 post-transplant in detecting delayed graft function (DGF), with an AUROC of 0.87 versus 0.56 for creatinine. • PenKid differentiates slow graft function (SGF) from DGF up to 8 days earlier than current methods, supporting more timely clinical decisions. • PenKid levels remain unaffected by kidney replacement therapy (KRT), allowing for more accurate assessment of kidney function. • Independent validation in transplant cohort from Australia confirms performance and broad applicability. Hennigsdorf/Berlin, Germany, July 1, 2025 - Diagnostic company SphingoTec GmbH (“SphingoTec”) announces a landmark study (1) published in Transplant International, led by Heidelberg University Hospital in Germany in collaboration with researchers from Sydney, Australia, which identifies Proenkephalin A 119-159 (penKid) as a reliable biomarker for early and precise assessment of graft function trajectories following kidney transplantation. The research demonstrates that PenKid not only identifies patients at risk for DGF significantly earlier than traditional markers but also distinguishes between slow and delayed graft function with remarkable accuracy, offering clinicians a valuable new tool for patient management. The study prospectively evaluated 159 consecutive kidney transplant recipients at Heidelberg University Hospital and validated findings in an independent cohort from Sydney. PenKid consistently outperformed serum creatinine (SCr) in predicting graft function trajectories, particularly in the critical early post-transplant period. Notably, PenKid’s ability to remain unaffected by KRT—a treatment for severe kidney dysfunction—further sets it apart from SCr, which can be influenced by non-renal factors and KRT itself, thereby enhancing the reliability of graft function assessment. Multivariate analysis confirmed PenKid as the strongest independent predictor of both short-term graft function and 30-day outcomes, underscoring its clinical utility for early risk stratification. The biomarker’s superior granularity allows for nuanced classification of DGF severity, supporting more informed decisions regarding the initiation of dialysis or biopsy and offering potential for individualized patient care. With these findings, penKid steps forward as a practical addition to the transplant clinician’s toolkit, promising to sharpen decision-making for optimal outcomes. Its adoption could help transplant teams act with greater confidence and precision, ultimately strengthening the standard of care in kidney transplantation. ## References 1. Benning L et al. (2025) Proenkephalin A 119-159 in Kidney Transplantation: A Novel Biomarker for Superior Tracking of Graft Function Trajectories. Transpl. Int. 38:14366. doi: 10.3389/ti.2025.14366 About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a biomarker company focusing on the out-licensing of innovative critical care solutions for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec develops its biomarkers to the commercial stage and partners with IVD companies to make them available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, commercially available on diagnostic platforms AFIAS and Nexus IB10 and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Discover more on www.sphingotec.com Contact : Ruxandra Lenz Marketing and Communication SphingoTec GmbH Phone +49-3302-20565-0 Email: press@sphingotec.com
• Boditech Med and SphingoTec announce the launch of the AFIAS sphingotest® penKid® assay, a diagnostic tool designed to aid in kidney function assessment for critically ill patients. • The IVDR-marked assay provides quantitative measurements of Proenkephalin A 119-159 (penKid), supporting clinical decision-making in acute and critical care. • This launch follows a licensing agreement granting Boditech the rights to develop and commercialize penKid-based assays, as well as a market development agreement between the two companies. Gangwon-do, Republic of Korea, and Hennigsdorf/Berlin, Germany, May 6, 2025 - Boditech Med Inc. (“Boditech”) and SphingoTec GmbH (“SphingoTec”) today announced the commercial launch of the AFIAS sphingotest® penKid® assay. The assay is designed to provide clinicians with a tool for assessing kidney function in critically ill patients by measuring Proenkephalin A 119-159 (penKid), a biomarker that reflects real-time kidney function independently of inflammation or comorbidities. The AFIAS sphingotest® penKid® assay is IVDR-marked for its intended use as an automated fluorescence immunoassay for the in vitro diagnostic quantitative determination of Proenkephalin A 119-159 in human whole blood/plasma, serving as an aid in the diagnosis of acute kidney injury (AKI) in adult patients with sepsis or septic shock. It provides additional information to support clinical decision-making in managing critically ill patients at risk of acute kidney injury (AKI). This launch is the result of a strategic collaboration between Boditech and SphingoTec, including a licensing agreement granting Boditech the rights to develop and commercialize penKid-based assays on its diagnostic platforms, as well as a market development agreement aimed at accelerating global commercialization efforts. Eui-Yul Choi, CEO of Boditech Med, commented: “We are proud to introduce the AFIAS sphingotest® penKid® assay to clinicians worldwide. This launch reflects our commitment to delivering innovative diagnostic solutions that address critical needs in healthcare. Our collaboration with SphingoTec has been instrumental in bringing this important tool to market.” Deborah Bergmann, CEO of SphingoTec, added: “The launch of AFIAS sphingotest® penKid® demonstrates how partnerships can drive innovation and improve access to advanced diagnostics. By combining our biomarker expertise with Boditech’s diagnostic capabilities, we are reaching a new chapter in our collaboration. We are streamlining our processes to support the business of our license partners and further expand our global reach. This enables us to provide clinicians with essential tools to enhance patient care.” Scientific insights on penKid PenKid is a biomarker that enables real-time assessment of kidney function (1). Unlike traditional markers such as serum creatinine, penKid levels are not influenced by inflammation or other confounding factors like age or sex (1,2,3). Studies have shown that penKid allows earlier detection of acute kidney injury (AKI), predicting changes in serum creatinine up to 48 hours before conventional diagnostic criteria are met (1,3). This early detection capability is particularly valuable in critically ill patients, including those with sepsis or septic shock (1,3,4). Additionally, penKid has shown potential for monitoring renal recovery under dialysis and could help predict successful weaning from renal replacement therapy (5,6). ## References 1. Hollinger A, et.al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int Rep. 2018 Aug 22;3(6):1424-1433. doi: 10.1016/j.ekir.2018.08.006. 2. Beunders et al. Assessing GFR With Proenkephalin, Kidney International Reports, 2023, DOI: https://doi.org/10.1016/j.ekir.2023.08.006 3. Caironi et al., Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem (2018) DOI:10.1373/clinchem.2018.288068 4. Moledina DG. Penkid: A Novel Biomarker of Reduced GFR in Sepsis. Kidney Int Rep. 2018 Nov 15;4(1):17-19. doi: 10.1016/j.ekir.2018.11.002. 5. von Groote T et al. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit Care 26, 333 (2022). doi.org/10.1186/s13054-022-04217-4 6. von Groote T, et al. Evaluation of Proenkephalin A 119-159 for liberation from renal replacement therapy: an external, multicenter pilot study in critically ill patients with acute kidney injury. Crit Care. 2023 Jul 10;27(1):276. doi: 10.1186/s13054-023-04556-w. About Boditech Boditech Med (based in Chuncheon, Gangwon-do, Republic of Korea) is a leading company in the field of point-of-care diagnostics, which has accumulated 25 years of business expertise in the field. The company has more than 80 types of in vitro diagnostic products that detect biomarkers related to infectious diseases, diabetes, cardiovascular diseases, cancer, and hormone-related diseases with its immunofluorescence lateral flow technology, quantitative immunofluorescence technology and spectrophotometric technology. And the list continues to grow with new high-value-added products. With its instrument platform installed in more than 120 countries, the company also has a stable revenue model. The company is currently strengthening its value as a global company by expanding its manufacturing bases in the US, China, India, and Indonesia. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a diagnostic company focusing on the out-licensing of innovative critical care biomarkers for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec develops its biomarkers to the commercial stage and partners with IVD companies to make them available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, commercially available on diagnostic platforms AFIAS and Nexus IB10 and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Discover more on www.sphingotec.com Contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Phone +49-3302-20565-0 Email: press@sphingotec.com
• Real-World Implementation: Proenkephalin A 119–159 (penKid) has been successfully integrated into daily practice in intensive care unit (ICU), demonstrating its effectiveness in assessing kidney function and predicting acute kidney injury (AKI) in over 4,000 patients. • Superior Dynamics: PenKid augments traditional markers by detecting rapid changes in kidney function, particularly beneficial in critically ill patients where timely information is crucial. • Clinical Impact: PenKid proved valuable in identifying high-risk patients at ICU admission, especially those with a normal serum creatinine. Additionally, penKid provided valuable insights into kidney function during renal replacement therapy (RRT). • International Recognition: Beyond its use in reference hospitals in Germany, international experts, including those at the Mayo Clinic, acknowledge the added value of penKid on top of standard care. Hennigsdorf/Berlin, Germany, April 16, 2025 - Diagnostic company SphingoTec GmbH (“SphingoTec”) announces the publication of real-world data on the effectiveness of Proenkephalin A 119–159 (penKid) in improving kidney function assessment in intensive care units (ICUs) (1). This study confirms penKid's value in identifying high-risk patients at admission and supporting the management of renal replacement therapy (1). The published data complement a recent review from the Mayo Clinic that highlights penKid's potential as a new method to measure renal function beyond creatinine (2). Acute kidney injury (AKI) is a significant challenge in intensive care units (ICUs), affecting up to half of all patients and leading to increased morbidity and mortality (1). Traditional markers like serum creatinine have limitations, as they may not detect kidney dysfunction until significant damage has occurred. A recent study from the University Hospital Aachen marks the first publication based on real-world data from over 4,000 patients, reporting the outcome implementation of penKid in daily practice. Study Overview During a two-year period, the University Hospital Aachen, Germany implemented penKid in their ICU (1). The study analyzed almost 18,000 penKid measurements from 4,169 patients, including the use of a penKid-GFR formula to estimate glomerular filtration rate (GFR). PenKid outperformed traditional markers in assessing kidney function and remained dynamic under RRT, supporting clinical decision-making in critical care. Key Findings The data show that penKid allows for a significantly better assessment of renal dysfunction upon admission, particularly in identifying patients at risk of developing severe AKI. In patients with normal serum creatinine levels at admission, penKid and its GFR formula outperformed traditional methods in predicting AKI within 24 and 48 hours. As penKid is effectively removed by RRT, as demonstrated by studies such as Lorenzin et al. (3), persistently elevated levels during RRT may indicate ongoing renal dysfunction, while declining levels could signal renal recovery. This dynamic nature of penKid – even during ongoing RRT - can support decisions that may optimize RRT duration and reduce associated risks. Clinical Significance Prof. Dr. Gernot Marx, the Director of the Clinic for Operative Intensive Care and Intermediate Care at Uniklinik RWTH Aachen highlights, "After using penKid in clinical settings for five years, I see significant value in specific use cases due to its swift dynamics, addressing creatinine blind spots. The published data confirms our clinical observations and paves the way for bringing innovation into daily practice." Early detection of compromised renal function could allow for timely interventions, reducing the risk of emergency RRT and improving the chance for full renal recovery. In RRT scenarios, penKid could help de-escalate treatments and prevent unnecessary RRT, potentially reducing ICU stays. Recent Review The critical care community is seeking tools for improving kidney health assessment, as indicated by a recent review published by researchers from the Mayo Clinic (2). This review highlights penKid's ability in detecting rapid GFR changes and predicting AKI, particularly in critically ill patients. PenKid's strong correlation with measured GFR makes it a valuable tool, enabling more accurate and timely assessments of kidney function. The Mayo Clinic review also notes that penKid's ability to predict major adverse kidney events, such as worsening renal function and the need for RRT, aligns with its potential to provide valuable prognostic information in clinical settings. ## References 1. Martin, L. et al. Implementation and One-Year Evaluation of Proenkephalin A in Critical Care. Int. J. Mol. Sci. 2025, 26, 2602. https://doi.org/10.3390/ijms26062602 2. Sheikh MS, Kashani KB. Beyond creatinine: New methods to measure renal function? Eur J Intern Med. 2025 Jan 31:S0953-6205(25)00025-1. doi: 10.1016/j.ejim.2025.01.015. 3. Lorenzin, A. et al. Human Proenkephalin A 119-159 (penKid) in Extracorporeal Therapies: Ex vivo Sieving Coefficient, Diffusive Clearance, and Hemoadsorption Kinetics. Blood Purif. 2024, 53, 773–780. doi: 10.1159/000540061. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a diagnostic company focusing on the out-licensing of innovative critical care biomarkers for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec develops its biomarkers to the commercial stage and partners with IVD companies to make them available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, commercially available on diagnostic platforms AFIAS and Nexus IB10 and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Discover more on www.sphingotec.com Contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Phone +49-3302-20565-0 Email: press@sphingotec.com
• Proenkephalin A 119-159 (penKid) demonstrates potential as a predictor for successful discontinuation of continuous renal replacement therapy (CRRT) in cardiac surgery patients. • Study reveals significant differences in penKid levels between patients successfully and unsuccessfully liberated from CRRT. • Findings suggest penKid could be a valuable tool in guiding CRRT liberation decisions. Hennigsdorf/Berlin, Germany, January 9, 2025 - Diagnostic company SphingoTec GmbH ("SphingoTec") announces results from the first prospective study, conducted at the Medical University of Vienna, demonstrating penKid as a discriminatory biomarker for successful liberation from CRRT in cardiac surgery patients with acute kidney injury (AKI) (1). AKI remains a significant challenge in hospitals and is not limited to emergency cases, affecting a substantial number of up to 40% of elective cardiac surgery patients (2,3). When prolonged or further complicated, the use of CRRT might become necessary, which is one of the most resource-intensive interventions. Determining the optimal time to discontinue CRRT has been a persistent clinical challenge. The research, led by Ap. Prof. PD DDr. Martin Bernardi and colleagues, provides new insights into the utility of this biomarker. Key Findings The study revealed that patients successfully liberated from CRRT had significantly lower penKid levels compared to those unsuccessfully liberated (1). This distinction was particularly notable at the time of CRRT liberation. "Our findings suggest that penKid could be a valuable tool in supporting clinical decisions regarding CRRT discontinuation," stated Prof. Bernardi. "Additionally, this biomarker shows potential in identifying patients who are likely to maintain kidney function after CRRT is stopped." Supporting evidence comes from post-hoc analyses of the RICH (5) and ELAIN (6) trials, which consistently demonstrate penKid's capability to predict kidney function recovery under renal replacement therapy and successful treatment liberation. Bridging Gaps in AKI Management As the critical care community continues to optimize AKI management protocols in cardiac surgery patients and beyond, penKid stands out as a promising biomarker that could refine current acute dialysis management strategies, particularly in determining optimal timing for CRRT discontinuation. For more information, Prof. Bernardi will be discussing these findings and their implications in an upcoming webinar titled "Practice-Oriented Management of Acute Dialysis in Cardiac Surgery Patients: Strategies and Challenges in Clinical Practice." ## References: 1. Tichy J, et.al. Prediction of Successful Liberation from Continuous Renal Replacement Therapy Using a Novel Biomarker in Patients with Acute Kidney Injury after Cardiac Surgery-An Observational Trial. Int J Mol Sci. 2024 Oct 10;25(20):10873. doi: 10.3390/ijms252010873. 2. Grams, M.E. et. al. Acute Kidney Injury after Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 872–880. 3. Wang, Y. et al. Cardiac surgery-associated acute kidney injury: Risk factors, pathophysiology and treatment. Nat. Rev. Nephrol. 2017, 13, 697–711. 4. Lorenzin A, et al. Human Proenkephalin A 119-159 (penKid) in Extracorporeal Therapies: Ex vivo Sieving Coefficient, Diffusive Clearance, and Hemoadsorption Kinetics. Blood Purif. 2024;53(10):773-780. doi: 10.1159/000540061. 5. von Groote T et al. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit Care 26, 333 (2022). doi.org/10.1186/s13054-022-04217-4 6. (3) von Groote T, et al. Evaluation of Proenkephalin A 119-159 for liberation from renal replacement therapy: an external, multicenter pilot study in critically ill patients with acute kidney injury. Crit Care. 2023 Jul 10;27(1):276. doi: 10.1186/s13054-023-04556-w. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a diagnostic company focusing on the out-licensing of innovative critical care biomarkers for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec develops its biomarkers to the commercial stage and partners with IVD companies to make them available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, commercially available on diagnostic platforms AFIAS and Nexus IB10 and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Discover more on www.sphingotec.com Contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Phone +49-3302-20565-0 Email: press@sphingotec.com

• Alliance marks first penKid licensing agreement for high throughput immunoassay. • Partnership leverages SphingoTec's proficiency in acute kidney injury testing, and Beckman Coulter’s extensive, globally installed Access Family of immunoassay analyzers. HENNIGSDORF/BERLIN, GERMANY — October 9, 2024: Diagnostic company SphingoTec GmbH (“SphingoTec”) today announced a new partnership with Beckman Coulter Diagnostics Inc. (“Beckman Coulter”). Through this collaboration, the companies will bring an assay for SphingoTec’s innovative kidney function biomarker, Proenkephalin 119-159 (penKid), to Beckman Coulter’s extensive test menu for use on the Access Family of Immunoassay Analyzers. This alliance marks the first central laboratory license for a penKid assay and aims to significantly enhance the diagnostic capabilities for acute kidney injury (AKI) globally, by leveraging Beckman Coulter’s global installed base of instruments. PenKid is a real-time biomarker in plasma designed to address critical gaps in the standard diagnostic practices for AKI, particularly in critical care environments. Specifically, scientific evidence shows that, unlike current methods, a penKid assay offers early detection of kidney function decline, unaffected by inflammation, potentially enabling earlier intervention and improved patient outcomes (1,2,3). The incidence of AKI is increasing in both hospital and community settings; it is estimated that more than 13 million people are affected by AKI annually worldwide (4, 5). Under the terms of the agreement, Beckman Coulter will develop and validate a fully automated diagnostic test for penKid, leveraging SphingoTec’s IVD certified assay. This assay has already been implemented as a routine test in the first university hospitals. This collaborative effort will facilitate high-throughput availability of penKid assays in central laboratories, supporting critical care physicians with the ability for timely and precise kidney health assessment. “Acute kidney injury exerts a profound impact on patients globally, complicates acute and chronic illnesses frequently resulting in poor outcomes, and rivals many other critical diseases in its severity and consequences,” said Kevin O’Reilly, President, at Beckman Coulter Diagnostics. “The published evidence for penKid testing combined with SphingoTec’s scientific expertise provides an exciting opportunity to improve kidney health management. Working with SphingoTec, our goal is to expand access to and improve workflow from this important kidney health innovation underscoring our commitment to enhancing patient care worldwide.” Deborah Bergmann, Managing Director and CEO of SphingoTec, emphasized, “The development of a penKid-based assay for use on Beckman Coulter’s globally installed immunoassay platforms represents a significant step toward realizing our vision of transforming diagnostic innovation into tangible patient benefits. This partnership accelerates our mission to deliver precise, actionable insights to clinicians worldwide, ultimately improving outcomes for patients suffering from acute kidney injury.” ### References: (1) Beunders et al. Assessing GFR With Proenkephalin, Kidney International Reports, 2023, DOI: https://doi.org/10.1016/j.ekir.2023.08.006 (2) Caironi et al., Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem (2018) https://doi.org/10.1373/clinchem.2018.288068 (3) Lin, LC., et al. Proenkephalin as a biomarker correlates with acute kidney injury: a systematic review with meta-analysis and trial sequential analysis. Crit Care 27, 481 (2023). https://doi.org/10.1186/s13054-023-04747-5 (4) Mehta RL et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015 Jun 27;385(9987):2616–43. http://dx.doi.org/10.1016/S0140-6736(15)60126-X (5) Susantitaphong P et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013 Sep;8(9):1482–93. http://dx.doi.org/10.2215/CJN.00710113 About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Discover more on www.sphingotec.com Contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Phone +49-3302-20565-0 Email: ruxandra.lenz@sphingotec.com

• SphingoTec GmbH has successfully secured funding to drive its global expansion, including the US market, through strategic collaborations. • The investment round is led by Think.Health Ventures, with support from existing investors. Think.Health Ventures will add significant value to SphingoTec through its expertise in diagnostics and its extensive network in clinics and laboratories. • The raised capital will be pivotal in advancing the out-licensing and commercialization of SphingoTec’s innovative biomarkers for critical care, aiming to significantly improve patient management and outcomes. • The funding underscores SphingoTec’s dedication to pioneering advancements in critical care patient management. Hennigsdorf/Berlin, Germany, August 8, 2024 - Diagnostics company SphingoTec GmbH (“SphingoTec”) announces the successful closing of a Series C financing round. The raised capital will allow the company to reach profitability through sustained and strategic commercialization of its products. This includes supporting market development, out-licensing activities, and close collaboration with licensees. SphingoTec aims for global expansion, including entry into the US market. The lead investor, Think.Health Ventures, specializes in healthcare ventures that focus on innovations and business models in digital healthcare, medical technologies, and health service providers. Think.Health Ventures will provide support to the SphingoTec management team through its extensive network in clinics and laboratories. Additionally, SphingoTec has secured support from existing investors, which include Brandenburg Kapital, HBM Healthcare Investments, NRW.BANK, and Wellington Partners. Dr. Florian Kainzinger, Managing Partner and Founder of Think.Health Ventures, commented, "We are pleased to support SphingoTec at this exciting stage of the company's development. With its developed and commercialized lead product penKid, SphingoTec is well-positioned for global expansion through out-licensing to key players in the diagnostic industry." SphingoTec develops and markets in vitro diagnostic (IVD) solutions for novel and proprietary blood-based protein biomarkers. The company focuses on translational medicine, bringing innovations to frontline healthcare by discovering biomarkers, developing IVD assays, and making them available on different diagnostic platforms. The biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function. PenKid has been out-licensed and commercialized and is currently used in routine measurements in university hospitals to improve patient management. To achieve broad market adoption of its first-in-class biomarkers, SphingoTec partners with global IVD companies. The portfolio is available for licensing to companies seeking to enhance their immunoassay portfolios and develop biomarker-based diagnostics for clinical use on high-throughput and near-patient testing platforms. Deborah Bergmann, Managing Director and CEO, stated, "There is a substantial demand for more advancements in critical care diagnostics. Our scientifically and clinically validated innovations have the potential to improve the management of critically ill patients. With this investment, we will focus on further out-licensing our biomarkers to translate scientific advancements into routine clinical practice, ultimately supporting clinicians in improving patient outcomes." ### About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. About penKid Proenkephalin A 119-159 (penKid) is a blood-based biomarker for assessing kidney function in acute and critical conditions. The biomarker offers a blood-based alternative for the complex and time-consuming in vivo measurement of true glomerular filtration rate (GFR). PenKid is independent of common comorbidities (e.g., hypertension and diabetes) and the frequently occurring inflammation in critically ill patients. Rising penKid blood levels predict acute kidney injury earlier than today’s standard of care, and decreasing penKid blood levels indicate the improvement of kidney function, even under dialysis. About bio-ADM Bioactive Adrenomedullin 1-52 (bio-ADM) is a dynamic blood biomarker for the real-time assessment of endothelial function. The endothelium contributes to blood pressure and separates blood from the surrounding tissue. Elevated bio-ADM blood levels predict both blood pressure drop, resulting in shock and leaky blood vessels, leading to edema formation. Early identification of an imbalance in endothelial function allows for the prediction of vasopressor demand in critically ill patients and aids in the identification of residual congestion in acute heart failure patients. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0

• SphingoTec and Boditech jointly introduced the kidney function biomarker Proenkephalin A 119-159 (penKid) at the 42nd AKI & CRRT course in Vicenza, highlighting its upcoming integration with the AFIAS platform. • PenKid is a real-time kidney function biomarker that addresses the standard acute kidney injury (AKI) diagnostic gaps. • During a scientific session on AKI biomarkers, penKid was presented as a biomarker for risk prediction and early identification of AKI, with performance data that could support clinical routine implementation for effective AKI management. Hennigsdorf/Berlin, Germany, June 18, 2024 - Diagnostics company SphingoTec GmbH (“SphingoTec”) introduced together with Boditech Med Inc. (Boditech) at the 42nd AKI & CRRT Course in Vicenza the innovative kidney function biomarker penKid developed by SphingoTec and licensed to Boditech. They highlighted the benefits of penKid on the AFIAS platform, emphasizing its promise in enhancing kidney function assessment. With the AFIAS penKid® test, the companies will focus on market development, engaging with healthcare professionals, and ensuring a successful product rollout starting in Europe. The current standard of care diagnosis for AKI is to use serum creatinine and urine output, which are both lagging indicators. Many clinical studies point to the delay in AKI identification and its impact on mortality. PenKid is a functional biomarker that overcomes the shortcomings in the standard AKI diagnosis of critically ill patients by assessing kidney function in real time without being influenced by inflammation. A new penKid-based formula for estimating the glomerular filtration rate (eGFR) has been developed, outperforming widely used conventional equations based on creatinine alone (1). In previous studies, penKid has shown the potential to support increased clinical vigilance by risk prediction and identifying sub-clinical AKI, with changes in penKid levels pointing to worsening or recovery of kidney function (2). Prof. Lui Forni, (Professor and Consultant in intensive care at Royal Surrey County Hospital NHS Foundation Trust and the School of Medicine, University of Surrey) introduced penKid to the international audience during the session “Update on biomarkers". In addition to the well-established scientific evidence on penKid as a kidney function biomarker for critical care settings, the presentation also included the results of a decentralized analysis that consolidates evidence from 11 independent studies and nearly 4,000 patients (3). Beyond unified and compelling evidence of penKid’s clinical performance, the systematic meta-analysis explores the role of penKid in identifying patients at high risk for AKI. Incorporating penKid into patient care could enable more intensive surveillance and personalized and early prevention efforts, including optimizing hemodynamic stability and prudent use of nephrotoxic agents. Prof. Lui Forni emphasizes the potential for penKid to serve as an alternative to serum creatinine in critical care: “While creatinine is the mainstay for monitoring kidney function in chronic kidney disease, its limitations in the acute setting call for better solutions. It's time to embrace novel diagnostics in our day-to-day practice.” ### References: (1) Beunders et al. Assessing GFR With Proenkephalin, Kidney International Reports, 2023, DOI: https://doi.org/10.1016/j.ekir.2023.08.006 (2) Caironi et al., Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem (2018) DOI:10.1373/clinchem.2018.288068 (3) Lin, LC., et al. Proenkephalin as a biomarker correlates with acute kidney injury: a systematic review with meta-analysis and trial sequential analysis. Crit Care 27, 481 (2023). https://doi.org/10.1186/s13054-023-04747-5 About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. About penKid Proenkephalin A 119-159 (penKid) is a blood-based biomarker for assessing kidney function in acute and critical conditions. The biomarker offers a blood-based alternative for the complex and time-consuming in vivo measurement of true glomerular filtration rate (GFR). PenKid is independent of common comorbidities (e.g., hypertension and diabetes) and the frequently occurring inflammation in critically ill patients. Rising penKid blood levels predict acute kidney injury earlier than today’s standard of care, and decreasing penKid blood levels indicate the improvement of kidney function, even under dialysis. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0

Hennigsdorf/Berlin, Germany, March 26, 2024 – Diagnostic company SphingoTec announces a significant change in its management team. Joerg Menten, Managing Director and CEO, leaves the company to enter into retirement. Dr. Angelo Moesslang, Managing Director and CFO, leaves SphingoTec after the completion of a 3-year term. SphingoTec is pleased to announce the following appointments to its management team, mainly selected from the internal ranks: • Deborah Bergmann as Managing Director and Chief Executive Officer (CEO) • Dr. Florian Uhle as Managing Director and Chief Medical Officer (CMO) • Nicole Witzmann as Chief Financial Officer (CFO) Dr. Gerald Moeller, Chairman of the Advisory Board at SphingoTec, said, "On behalf of the Advisory Board, I would like to thank Joerg Menten and Dr. Angelo Moesslang for successfully leading the company during the past years in a challenging external environment, preparing SphingoTec for entering into first commercial agreements, and streamlining the company's complex portfolio. This will allow their successors to focus on the key value drivers going forward. Furthermore, I congratulate Deborah Bergmann, Dr. Florian Uhle, and Nicole Witzmann on their appointments. Their hands-on experience in our global industry will help grow our company to become a key provider of diagnostic innovations in critical care. The long-term and multi-faceted past management roles of Deborah Bergmann within the company offer an experienced and dynamic leader. Dr. Florian Uhle is completing the team with his deep understanding of critical care settings and experience in translating science into clinical routine practice, and Nicole Witzmann with her successful track record in ensuring financing structure, fundraising, and allocation of funds for successfully achieving relevant milestones. I look forward to dynamic and focused actions with the new management team." Deborah Bergmann, CEO of SphingoTec, stated, "With gratitude to the outgoing management team for their dedicated leadership, I am honored by this appointment and committed to building upon their foundation. Together with the team, we will expand the global reach of our biomarker innovations, focusing on enhancing patient care and addressing unmet clinical needs. Our collective effort aims to reshape diagnostic standards in critical care while focusing on further licensing agreements and assisting current partners and pharma cooperations. Additionally, we are committed to supporting existing customers as routine users, particularly university hospitals, and strengthening the scientific and clinical evidence around our diagnostic tests." Deborah Bergmann appointed as Managing Director and CEO: Deborah Bergmann brings a wealth of experience and a deep understanding of SphingoTec's mission. With a Master's degree in Biochemistry from the University of Potsdam, Ms. Bergmann has been an integral part of SphingoTec's journey from its inception. Her multifaceted contributions across research and development, marketing, sales, and business development have been instrumental in securing the company's first routine users, driving sales conversion of reference customers, and overseeing the out-licensing activities. Ms. Bergmann's leadership skills and international cooperation aptitude have facilitated the company's expansion into global markets, underscoring her commitment to advancing diagnostic standards in critical care. Dr. Florian Uhle appointed as Managing Director and CMO: Dr. Florian Uhle brings over 15 years of experience in healthcare. With a diploma in Biology from the Justus-Liebig University, a Master of Science in Clinical Research and Translational Medicine from the University of Leipzig, and a Ph.D. in Translational Critical Care from the Justus-Liebig University, Dr. Uhle has held several leadership roles within Inflammatix Inc. and the University Hospitals Heidelberg and Giessen. His expertise in diagnostic research activities and innovation-oriented initiatives, coupled with his extensive publication record in renowned scientific journals, positions him as a valuable leader to SphingoTec. Dr. Uhle is committed to bridging the gap between research and clinical practice, ensuring that SphingoTec's diagnostic innovations translate into tangible improvements in patient outcomes. Nicole Witzmann joins SphingoTec as CFO: Nicole Witzmann assumes the role of Chief Financial Officer, bringing over eight years of corporate finance experience in the healthcare industry. With a Master's degree in Business Communication from the University of Applied Sciences Berlin (HTW Berlin), Ms. Witzmann previously served as CFO at InfanDx. She was instrumental as Head of Finance at Adrenomed AG, ensuring financial oversight and fundraising. Her expertise aligns seamlessly with SphingoTec's strategic objectives: allocating financial resources, which aims to achieve tangible milestones, fostering shareholder value, and substantial advancements in patient care. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0
• PenKid is a kidney function biomarker that correlates with gold standard GFR measurements in stable and acute kidney injury (AKI) patients. • Based on penKid, a new formula was developed to estimate the GFR, outperforming widely used conventional equations based on creatinine alone. • Scientists from Radboud University and Mayo Clinic developed and validated this formula in a large cohort of stable, as well as critically ill patients, laying the foundation for future increased clinical vigilance in kidney health assessment. Hennigsdorf/Berlin, Germany, October 5, 2023 - Diagnostic company SphingoTec GmbH (“SphingoTec”) announces that a new, improved formula has been developed for estimating the GFR using its kidney function biomarker penKid (1). According to the data, penKid strongly correlates with the measured GFR (mGFR), while the penKid-based formula for estimating GFR performs better than routinely used equations. In clinical practice, kidney dysfunction is monitored using creatinine-based estimates of GFR. Creatinine is recognized as a late and insensitive biomarker of GFR, with consensus guidelines emphasizing that a more timely and accurate estimation of GFR in AKI is a relevant unmet medical need (2,3,4). Researchers from the Radboud University Medical Centre, Netherlands, and Mayo Clinic, USA, have included a cohort of over 1,300 patients in a data-driven approach for developing an equation to estimate the GFR using the kidney function biomarker penKid (1). According to the findings, this novel equation performs better in calculating eGFR compared to creatinine-based equations. The formula can be applied to a wide range of patients since the cohort used for its development and validation included a broad spectrum of patients, such as patients with established chronic kidney disease, and critically ill patients. The compelling data is further strengthened by the fact that gold-standard methods to determine the true mGFR were used in all patients, and penKid concentration was most strongly correlated with mGFR. Prof. Peter Pickkers (Head of Research in Intensive Care Medicine at Radboud University Nijmegen Medical Centre) summarized, “The innovative biomarker penKid has previously demonstrated significant potential in increasing the clinical vigilance and supporting the management of AKI patients. The penKid-based formula will facilitate in future its implementation in the clinical routine since it translates penKid concentrations into a more accurate GFR estimation that can now be implemented by physicians worldwide.“ Dr. Florian Uhle, Medical Director at SphingoTec, added, “With this landmark research, the first formula using penKid has been successfully established in chronic and acute kidney impairment. Starting from this evidence base, we will focus on further validating this formula in critically ill patients and aiming to overcome the shortcomings of our current diagnostic standards in AKI management. We are looking forward to interacting closely with global healthcare providers in the future to drive this concept to clinical reality.” ## References: (1) Beunders et. al. Assessing GFR With Proenkephalin, Kidney International Reports, 2023, DOI: https://doi.org/10.1016/j.ekir.2023.08.006 (2) Chawla et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nature Reviews Nephrology 2017; 13: 241-257. (3) Ostermann et al. Report of the first AKI Round Table meeting: an initiative of the ESICM AKI Section. Intensive Care Med Exp 2019; 7: 69. (4) Pickkers et al. The intensive care medicine agenda on acute kidney injury. Intensive care medicine 2017; 43: 1198-1209. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for diagnosing, predicting, and monitoring acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. About penKid Proenkephalin A 119-159 (penKid) is a blood-based biomarker for assessing kidney function in acute and critical conditions. The biomarker offers a blood-based alternative for the complex and time-consuming in vivo measurement of true glomerular filtration rate (GFR). PenKid is independent of common comorbidities (e.g., hypertension and diabetes) and the frequently occurring inflammation in critically ill patients. Rising penKid blood levels predict acute kidney injury earlier than today’s standard of care, and decreasing penKid blood levels indicate the improvement of kidney function, even under dialysis. Scientific evidence shows that penKid also reflects kidney function in children, representing a potential biomarker for pediatric AKI. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0

• International experts recognize the need for diagnostic innovations to support more informed and standardized decisions for liberation from renal replacement therapy (RRT). • A presentation during the 41st Vicenza Course on AKI-CRRT highlights the promising results of a real-world evaluation of kidney function under RRT with Proenkephalin A 119-159 (penKid). • Recently published scientific data affirms that penKid remains informative under acute renal replacement therapy (RRT) and can identify patients for successful liberation. Hennigsdorf/Berlin, Germany, September 6, 2023 – Diagnostic company SphingoTec has announced that a new study and real-world data on its kidney function biomarker penKid underline its potential to monitor kidney function during acute kidney injury (AKI) and RRT and to support liberation decisions from RRT. These findings are especially relevant in the context of a liberation failure rate of up to 50% (1), considering the lack of consensus guidelines, and tools to support clinical decision-making. Experts advocate for improved tools in critical decision-making RRT, a common practice in Intensive Care Units (ICUs), has long relied on clinical observations for stop decisions. However, the lack of a dependable biomarker poses challenges in accurately assessing when to discontinue RRT. Considering this, leading experts from the field have discussed in a recent advisory board the importance of identifying the right tools to improve the outcomes in AKI. According to Claudio Ronco (Director of the International Renal Research Institute of Vicenza, Italy), "The future availability of a reliable biomarker to support stop decisions in RRT is crucial. It has the potential to minimize liberation failure rates and enhance patient care significantly." Lui Forni (Professor and Consultant in intensive care at Royal Surrey County Hospital NHS Foundation Trust and the School of Medicine, University of Surrey) highlights the ethical concerns surrounding unsuccessful liberation, stating, "When patients need to restart RRT after failed liberation, in many cases this will affect the overall patient outcome with an increase in mortality being seen. Therefore, it is imperative that we explore solutions that can help avoid such scenarios." The expert group supports the planning of a prospective study involving penKid as a potential tool to promote uniform treatment standards for RRT liberation. Advancements in assessing kidney function under RRT During the 41st Vicenza Course on AKI-CRRT, a presentation by Christian Nusshag (Senior Physician at the University Hospital Heidelberg, Germany) and titled “Real-world evaluation of residual kidney function under RRT - what’s new?” introduced the latest insights emerging from real-world data. Dr. Nusshag highlighted, "In the realm of AKI, there is a recognized need for relevant biomarkers that can accurately assess kidney function, especially in the context of RRT. The data we generated with penKid during our real-world evaluation at University Hospital Heidelberg, allowed us to observe that penKid seems not to be affected by RRT procedure, as standard markers are. In this case, penKid may help to identify patients at high risk for liberation failure or patients with unnecessary prolongation of RRT, potentially supporting successful RRT liberation decisions in the future." Recently published data support these real-world findings. The post-hoc analysis of the multicentric RICH trial show that penKid may be a competent biomarker to monitor the recovery of kidney function during continuous RRT (CRRT) and identify patients for liberation from CRRT (2). This aligns with data from the ELAIN trial, where penKid showed the potential to guide early and successful liberation of treatment (3). Dr. Florian Uhle, Medical Director at SphingoTec comments, “PenKid’s ability to provide patient-specific insights for informed clinical decision-making opens exciting possibilities in monitoring kidney function in critically ill patients, as it mirrors the real-time glomerular filtration rate in non-stable settings. The potential of penKid in kidney health assessment opens new avenues for personalized medicine in AKI.” ## References: (1) Schiffl H, et al. Current Approach to Successful Liberation from Renal Replacement Therapy in Critically Ill Patients with Severe Acute Kidney Injury: The Quest for Biomarkers Continues. Mol Diagn Ther. 2021 Jan;25(1):1-8. doi: 10.1007/s40291-020-00498-z. (2) von Groote T et al. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit Care 26, 333 (2022). https://doi.org/10.1186/s13054-022-04217-4 (3) von Groote T, et al. Evaluation of Proenkephalin A 119-159 for liberation from renal replacement therapy: an external, multicenter pilot study in critically ill patients with acute kidney injury. Crit Care. 2023 Jul 10;27(1):276. doi: 10.1186/s13054-023-04556-w. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for the diagnosis, prediction, and monitoring of acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0
• In 2022, Boditech Med and SphingoTec closed a global licensing agreement to develop and offer the Proenkephalin A 119-159 (penKid) test on Boditech's automated immunoassay systems. • The current market development agreement aims at enhancing the commercialization of the upcoming assay for penKid and ultimately advancing AKI diagnostics. Gangwon-do, Republic of Korea and Hennigsdorf/Berlin, Germany, August 31, 2023 - Boditech Med Inc. (“Boditech”) and SphingoTec GmbH (“SphingoTec”) today announced they have entered into a market development agreement (MDA), building on their previous licensing collaboration for the kidney function biomarker penKid. The MDA solidifies the partnership between Boditech and SphingoTec, aiming to support the successful commercialization of the assay for the biomarker penKid on the AFIAS platforms. This strategic collaboration unites the expertise and resources of both companies to drive advancements in kidney health assessment, addressing the unmet needs in acute kidney injury (AKI) diagnosis. AKI is a life-threatening condition that affects 13 million patients worldwide (1), and existing diagnostic standards often fall short, particularly for non-stable patients (2). The biomarker penKid shows significant potential to address these unmet needs as it delivers information on kidney function earlier and more precisely than the currently used markers, even under renal replacement therapy (3,4). Eui-Yul Choi, CEO, Boditech stated, “We are excited to soon introduce this innovative diagnostic biomarker to the market through our point-of-care platforms. To expedite market development, we have made the strategic decision to collaborate with SphingoTec, leveraging their know-how and experience in working alongside a global network of medical experts.” SphingoTec's CEO, Jörg Menten, emphasized on the significance of the partnership saying “We are dedicated to expanding our collaboration and providing robust support for the commercialization of penKid. By combining expertise, resources, and a shared vision, both companies are committed to advance AKI diagnostics and improve the management of critically ill patients.” ## References (1) Ponce D, et al. Acute kidney injury: risk factors and management challenges in developing countries. Int J Nephrol Renovasc Dis. 2016 Aug 22;9:193-200. doi: 10.2147/IJNRD.S104209. PMID: 27578995; PMCID: PMC5001661 (2) Makris K, et al. Acute Kidney Injury: Diagnostic Approaches and Controversies. Clin Biochem Rev. 2016 Dec;37(4):153-175. PMID: 28167845; PMCID: PMC5242479. (3) Hollinger A, et al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int Rep. 2018 Aug 22;3(6):1424-1433. doi: 10.1016/j.ekir.2018.08.006. (4) von Groote et al. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit Care 26, 333 (2022). doi.org/10.1186/s13054-022-04217-4 About Boditech Boditech Med (based in Chuncheon, Gangwon-do, Republic of Korea) is a leading company in the field of point-of-care diagnostics, which has accumulated 25 years of business expertise in the field. The company has more than 80 types of in vitro diagnostic products that detect biomarkers related to infectious diseases, diabetes, cardiovascular diseases, cancer, and hormone-related diseases with its immunofluorescence lateral flow technology, quantitative immunofluorescence technology and spectrophotometric technology. And the list continues to grow with new high-value-added products. With its instrument platform installed in more than 120 countries, the company also has a stable revenue model. The company is currently strengthening its value as a global company by expanding its manufacturing bases in the US, China, India, and Indonesia. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for the diagnosis, prediction, and monitoring of acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. About penKid Proenkephalin A 119-159 (penKid) is a blood-based biomarker for assessing the kidney function in acute and critical conditions. The biomarker offers a blood-based alternative for the complex and time-consuming in vivo measurement of true glomerular filtration rate (GFR). PenKid is independent of common comorbidities (e.g. hypertension and diabetes) and the frequently occurring inflammation in critically ill patients. Rising penKid blood levels predict acute kidney injury earlier than today’s standard of care and decreasing penKid blood levels indicate the improvement of kidney function. Scientific evidence shows that penKid also reflects kidney function in children, representing a potential biomarker for pediatric AKI. Press contact Boditech Med Inc. Duk Seung Na Email: nds@boditech.co.kr Phone: +821028451778 www.boditech.co.kr SphingoTec GmbH Ruxandra Lenz Email: press@sphingotec.de Phone: +49-3302-20565-0 www.sphingotec.com

• SphingoTec GmbH has received its IVDR certificate for its sphingotest® penKid® confirming the quality and compliance of this product with regulatory requirements. • Sphingotest® penKid® aids in the diagnosis of acute kidney injury and meets rigorous safety standards to ensure the best possible outcomes for critically ill patients. Hennigsdorf/Berlin, Germany, May 9, 2023 - Diagnostic company SphingoTec has been successfully re-certified according to ISO 13485 and received its first IVDR certificate for its IVD product sphingotest® penKid®. The certificates awarded by its Notified Body TÜV SÜD Product Service GmbH demonstrate the commitment of SphingoTec to meet the high-quality requirements of the ISO 13485 and the (EU) 2017/746 (IVDR). The IVDR-compliant and CE IVD-released product sphingotest® penKid® is used as an aid in the diagnosis of kidney injury in patients with sepsis or septic shock and meets the regulatory requirements to ensure the safety of critically ill patients to the utmost extent possible. Dr. Angelo Moesslang, Managing Director and CFO of SphingoTec commented, “Ensuring compliance in all aspects of our clinical biomarker validation processes is an absolute requirement for our company and our licensing partners. This includes robust quality management, setting up and maintaining technical documentation, continuous biomarker evaluation, and diligent manufacturing of products. By maintaining these quality standards, we are able to offer our partners valuable business opportunities and a strong competitive advantage in the marketplace.” As a company committed to innovation in the field of diagnostic testing, SphingoTec plans to continue collaborating with official regulatory bodies in the future to certify further products and expand existing certifications. By building up on the success with sphingotest® penKid®, SphingoTec aims to lead the way in developing diagnostic tools that meet the highest standards of quality, safety, and regulatory compliance. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for the diagnosis, prediction, and monitoring of acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0

• The Acute Disease Quality Initiative (ADQI) has published a consensus statement that strongly recommends using innovative biomarkers to improve the management of sepsis-associated acute kidney injury (SA-AKI). • According to the statement, the kidney function biomarker Proenkephalin A 119-159 (penKid) can detect subclinical AKI, enabling early risk prediction for upcoming AKI. • Looking ahead, integrating clinical information including relevant biomarkers might support the identification of specific disease endotypes and organ-related tolerance mechanisms as a basis for future personalized therapies. Hennigsdorf/Berlin, Germany, April 4, 2023 - Diagnostic company SphingoTec announces that in the consensus statement of the 28th ADQI workgroup, penKid is recognized as a relevant functional biomarker able to predict SA-AKI with high accuracy (1). Sepsis is a life-threatening disease that leads to organ dysfunction, accounting for up to 70% of all cases of AKI in critically ill patients (1). The current standard of care diagnostics for AKI has considerable limitations, therefore it is an urgent need to implement new biomarkers to enable better patient management. According to the consensus statement, penKid can identify patients with sepsis who are at an increased risk of developing AKI and major adverse kidney events (1). The study cites previous research that demonstrates the ability of penKid to detect subclinical AKI, including multicentric studies that shows the incidence and outcome relevance of subclinical AKI in critically ill patients (2,3). SA-AKI is a heterogeneous syndrome, with multiple mechanisms that contribute to its development. The recommendations also include the use of biomarkers and clinical information to identify distinct disease mechanisms and characteristics that can inform personalized treatment decisions in clinical practice. Furthermore, the same granular approach focused on early patient risk identification could also enable predictive enrichment in randomized trials of development-stage therapeutics. Dr. Florian Uhle, Medical Director at SphingoTec commented “There are few options available for assessing the kidney function in the context of AKI, and the standard of care biomarkers have well-known limitations, especially in septic patients. PenKid offers additional insights that could help clinicians recognize subclinical AKI and take preventive measures before the true problem even occurs. Since the main therapy in AKI relies on the management of symptoms, there is also a high need to advance the drug development and use these tools to support the identification of the right patients with the highest benefit and no harm in clinical trials.” Beyond the early detection of SA-AKI, previous scientific evidence shows that penKid has other applications that make it a versatile biomarker for kidney function assessment. PenKid correlates to the true glomerular filtration rate (true GFR), detects the presence and severity of AKI, identifies patients at high risk of unfavorable outcomes, and indicates renal recovery, even under dialysis (3,4). ### References: 1. Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol (2023). https://doi.org/10.1038/s41581-023-00683-3 2. Depret, F. et al. Incidence and outcome of subclinical acute kidney injury using penKid in critically ill patients. Am. J. Respir. Crit. Care Med. 202, 822–829 (2020). 3. Hollinger, A. et al. Proenkephalin A 119-159 (Penkid) is an early biomarker of septic acute kidney injury: the kidney in sepsis and septic shock (Kid-SSS) study. Kidney Int. Rep. 3, 1424–1433 (2018). 4. von Groote T et al. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit Care 26, 333 (2022) About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for the diagnosis, prediction, and monitoring of acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0

Hennigsdorf/Berlin, Germany, February 23, 2023 – Diagnostic company SphingoTec is pleased to announce the appointment of Dr. Florian Uhle as the company's new Medical Director. Dr. Uhle is a subject matter expert in translating innovations into routine clinical practice with a broad experience in both clinical and corporate settings. Dr. Uhle brings a wealth of knowledge and experience to the role, having spent 15 years in healthcare. In his new position, he will work cross-functionally to perfect the clear applications of the company's innovative diagnostic biomarkers while focusing on expanding their use for added value in clinical practice. SphingoTec recognizes the importance of addressing unmet needs in critical care settings with clear applications that can improve decision-making and patient management while reducing healthcare cost. Jörg Menten, Managing Director and CEO of SphingoTec, comments, "We are thrilled to welcome Dr. Uhle to our team as our new Medical Director. His experience in the medical field and passion for innovation make him the perfect candidate for this role. He will support our goal of increasing awareness and demand for our biomarker-based diagnostic innovations within the acute and critical care community." Dr. Florian Uhle said, "I am excited to join SphingoTec and work with such a talented and innovative team. The company's diagnostic portfolio has the potential to meet the growing need for effective diagnostic tools in critical care settings. I am eager to apply my knowledge and expertise to help bring the company's biomarkers to the forefront of clinical practice and to work towards improving patient outcomes." Before joining SphingoTec, Florian Uhle, Ph.D., was Director of Medical Affairs at Inflammatix Inc., a development-stage diagnostic company focusing on acute and critically ill patients. Prior to that, he held several leadership roles within the University Hospitals Heidelberg and Giessen, focusing on leading diagnostic research activities and innovation-oriented initiatives. He has a diploma in Biology from the Justus-Liebig University, a Master of Science from the University of Leipzig in Clinical Research and Translational Medicine, and a Ph.D. in Human Biology from the Justus-Liebig University. He completed the International Graduate School „Molecular Biology and Medicine of the Lung“ at the Universities of Giessen and Marburg Lung Center. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for the diagnosis, prediction, and monitoring of acute medical conditions. SphingoTec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function in critical diseases, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function in conditions like sepsis. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0
• PenKid identified as a promising kidney function biomarker to support future precision medicine approaches for RRT-dependent AKI. • A post-hoc analysis of the ELAIN trial indicates that penKid could enable the assessment of kidney function under RRT and guide early and successful liberation of treatment. • For the risk stratification of AKI patients, penKid may be a useful biomarker in identifying the need for timely RRT initiation. Hennigsdorf, Germany, December 15, 2022 - The diagnostic company SphingoTec GmbH (SphingoTec) announces first data on the kidney function biomarker penKid as a promising tool in supporting early initiation and liberation from RRT (1). Although RRT remains a pivotal measure in treating AKI complications, it is also associated with significant risks and its duration should be restricted to a minimum. The current findings show that penKid has the potential to reveal unprecedented insights on the underlying kidney function prior and during ongoing RRT, facilitating an early and successful termination of treatment. This may support timely risk stratification and individualization of treatment pathways. To allow kidney recovery in critically ill patients with AKI, RRT remains the key rescue therapy. However, no precise prediction tools are currently available to determine RRT liberation, although 5 - 13% of AKI patients have a high need for RRT (2,3). Due to scarcely available options, discontinuation of RRT relies on expert-based recommendations or clinical judgment based on controversial indications such as urine output. And yet, early discontinuation of RRT would not only be beneficial for the patient but also support the allocation of limited intensive care resources, thereby helping to reduce costs of unnecessarily long periods of RRT or unsuccessful termination with subsequent need for re-initiation of RRT (4). The current analysis has demonstrated that penKid could be a competent marker to monitor kidney function during ongoing RRT (1). Low penKid values during RRT were associated with earlier and successful liberation from RRT, compared to the group presenting high penKid levels. As a consequence, penKid could support clinicians identify those patients with sufficient kidney recovery, suitable for RRT liberation. In addition, at the time point of AKI diagnosis, measuring penKid values could allow the identification of patients who may benefit from early RRT initiation. Supplementary data suggest that prompt intervention may result in shorter RRT duration. The results of this study are complemented by previous scientific evidence showing that penKid mirrors real-time glomerular filtration rate (GFR) even in non-stable settings (5). Furthermore, penKid is unaffected by non-renal factors such as inflammation, common comorbidities, and gender (6,7,8), making it a suitable biomarker, especially for critically ill patients. Deborah Bergmann, Director of Marketing and Sales at SphingoTec commented, “The implications of the current findings are of great significance for future individualized approaches in managing RRT-dependent AKI. For the first time, scientists identified a biomarker that has the potential to shed light on clinically hidden developments in patients undergoing RRT, a highly challenging stage. This may benefit patients’ health on short and long term, as well as support healthcare providers reduce costs and better assign resources.” ## References: 1. von Groote T et al. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit Care 26, 333 (2022). https://doi.org/10.1186/s13054-022-04217-4 2. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. 3. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8. 4. Korkeila M, Ruokonen E, Takala J. Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med. 2000;26:1824-1831. 5. Beunders R, van Groenendael R, Leijte G, Kox M, Pickkers P. Proenkephalin compared to conventional methods to assess kidney function in critically ill sepsis patients. Shock. 2020 Jan;1 6. Beunders et al. (2017), Proenkephalin (PENK) as a novel biomarker for kidney function, JALM, DOI: 10.1373/jalm.2017.023598 7. Caironi P, Latini R, Struck J, Hartmann O, Bergmann A, Bellato V, et al. Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem. 2018;64((9)):1361–9. 8. Donato LJ, Meeusen JW, Lieske JC, Bergmann D, Sparwaßer A, Jaffe AS. Analytical performance of an immunoassay to measure proenkephalin. Clin Biochem. 2018 Aug;58:72–7. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. Sphingotec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for assessment of endothelial function in conditions like sepsis, and Proenkephalin A 119-159 (penKid), a biomarker for assessment of kidney function in critical diseases. About penKid Proenkephalin A 119-159 (penKid) is a blood-based biomarker for assessing the kidney function in acute and critical conditions. The biomarker offers a blood-based alternative for the complex and time-consuming in vivo measurement of true glomerular filtration rate (GFR). PenKid is independent of common comorbidities (e.g. hypertension and diabetes) and the frequently occurring inflammation in critically ill patients. Rising penKid blood levels predict acute kidney injury earlier than today’s standard of care and decreasing penKid blood levels indicate the improvement of kidney function. Scientific evidence shows that penKid also reflects kidney function in children, representing a potential biomarker for pediatric AKI. Media contact: Ruxandra Lenz Head of Marketing and Communication SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0

• Worldwide licensing agreement signed to develop and offer penKid as test on Boditech’s fully automated immunoassay systems. • The biomarker penKid adds diagnostic value and addresses current unmet needs in the management of patients suffering from acute kidney injury. • PenKid’s availability on Boditech’s global network of analyzers will amplify the reach of penKid to the wider critical care community. Gangwon-do, Republic of Korea and Hennigsdorf/Berlin, Germany, November 15, 2022 - Boditech Med Inc. (“Boditech”) and SphingoTec GmbH (“SphingoTec”) today announced they have entered into a non-exclusive royalty-bearing license agreement. Under the terms of this agreement, Boditech has obtained the rights to develop and commercialize clinical tests for the kidney function biomarker penKid on its renowned AFIAS and ichroma Point of Care platforms. Through this partnership, Boditech and SphingoTec will make the biomarker penKid available on Boditech’s worldwide installed base with the goal of improving the management of patients suffering from acute kidney injury (AKI). AKI affects one in five hospitalized patients (1). The critical state is currently diagnosed by standard-of-care biomarkers when 50% of the kidney function is already lost (1). PenKid addresses these pitfalls, offering an earlier and more precise determination of kidney function in acute and critical care settings (2). Eui-Yul Choi, CEO, Boditech Med said “Expanding our critical care portfolio with innovation in the field of acute diseases can, in the future, support our customers applying medical advancements and improve patient outcomes. After successfully evaluating the feasibility of penKid on our diagnostic solutions, we are looking forward to working with SphingoTec for the development and commercialization of the new test.” Jörg Menten, CEO, SphingoTec said “Boditech is a leader in point of care diagnostics with a strong global footprint. The agreement entered into with Boditech marks a significant milestone in SphingoTec’s mission to provide early detection of acute kidney injury to the international acute and critical care community. Our scientific research shows the future potential of penKid to monitor kidney function in further clinical settings such as renal replacement therapy (3) and pediatric AKI management (4,5). We are excited to work with our new partner to bring these advancements to critical care physicians around the globe very soon.” ## References: 1. Kellum, J.A. et al. Acute kidney injury. Nat Rev Dis Primers 7, 52 (2021). https://doi.org/10.1038/s41572-021-00284-z 2. Hollinger A, et al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int Rep. 2018 Aug 22;3(6):1424-1433. doi: 10.1016/j.ekir.2018.08.006. 3. von Groote et al. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: a post hoc analysis of the ELAIN trial. Crit Care 26, 333 (2022). https://doi.org/10.1186/s13054-022-04217-4 4. Smeets NJL, et al. Proenkephalin A as a marker for glomerular filtration rate in critically ill children: validation against gold standard iohexol GFR measurements. Clin Chem Lab Med. 2022 Oct 27. doi: 10.1515/cclm-2022-0545. 5. Hartman et al (2020), Proenkephalin as a New Biomarker for Pediatric Acute Kidney Injury - Reference Values and Performance in Children Under One Year of Age, Clin Chem Lab Med, doi: 10.1515/cclm-2020-0381 About Boditech Boditech Med (based in Chuncheon, Gangwon-do, Republic of Korea) is a leading company in the field of point-of-care diagnostics, which has accumulated more than 20 years of business expertise in the field. The company has more than 85 types of in vitro diagnostic products that detect biomarkers related to infectious diseases, diabetes, cardiovascular diseases, cancer, and hormone-related diseases with its immunofluorescence lateral flow technology, quantitative immunofluorescence technology and spectrophotometric technology. And the list continues to grow with new high-value-added products. With its instrument platform installed in more than 120 countries, the company also has a stable revenue model. The company is currently strengthening its value as a global company by expanding its manufacturing bases in the US, China, India, and Indonesia. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) is a commercial-stage diagnostic company focusing on innovative critical care biomarker for the diagnosis, prediction and monitoring of acute medical conditions. Sphingotec’s innovative markers are made available on different IVD platforms. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for assessment of endothelial function in conditions like sepsis, and Proenkephalin A 119-159 (penKid), a biomarker for assessment of kidney function in critical diseases. About penKid Proenkephalin A 119-159 (penKid) is a blood-based biomarker for assessing the kidney function in acute and critical conditions. The biomarker offers a blood-based alternative for the complex and time-consuming in vivo measurement of true glomerular filtration rate (GFR). PenKid is independent of common comorbidities (e.g. hypertension and diabetes) and the frequently occurring inflammation in critically ill patients. Rising penKid blood levels predict acute kidney injury earlier than today’s standard of care and decreasing penKid blood levels indicate the improvement of kidney function. Scientific evidence shows that penKid also reflects kidney function in children, representing a potential biomarker for pediatric AKI. Press contact Boditech Med Inc. Duk Seung Na Email: nds@boditech.co.kr Phone: +821028451778 www.boditech.co.kr SphingoTec GmbH Ruxandra Lenz Email: press@sphingotec.de Phone: +49-3302-20565-0 www.sphingotec.com
Acute kidney injury (AKI) is a complex syndrome, which results in a rapid loss of kidney function, affecting 1 in 5 patients (1) during hospitalization. Four units at the University Hospital Heidelberg and Heidelberg Kidney Center implement Proenkephalin (penKid) in a pilot biomarker-based treatment approach to diagnose AKI faster and monitor it more accurately across departments. PenKid is a proposed biomarker for real-time kidney function that potentially closes on the gaps of the standard diagnostic procedures. Hennigsdorf, Germany, August 26, 2022 - The diagnostic company SphingoTec GmbH (SphingoTec) announces the introduction of novel biomarker penKid in the clinical routine management of patients on a test basis in a total of four units at the University Hospital Heidelberg (UKHD) and Heidelberg Kidney Center (NZH). This includes the normal, the intermediate care (IMC), the nephrology, and the intensive care units (ICU) of both clinics in Heidelberg. According to recent data, the new diagnostic tool provides a faster and more accurate assessment of kidney function than current practice and aims to improve the management of patients with AKI. The condition is associated with high morbidity and mortality, and is a frequent complication of severe diseases, such as sepsis. The pilot implementation of penKid was started in cooperation with the Clinic for Anesthesiology at UKHD and the NZH with the long-term goal of improving the monitoring of patient’s kidney function. This routine use of penKid on a centralized level in the hospital drives innovation on multiple wards such as nephrology, ICU and IMC. The biomarker will be used on top of standard of care, in order to compare penKid with the existing standard diagnostics. Better and faster information assessment of kidney function helps detect AKI earlier, so that prompt action can be taken to protect renal function and improve patient prognosis. Prof. Dr. med. Martin Zeier, Medical Director at NZH added, “After the first experience of penKid on the point of care analyzer from SphingoTec, we have decided to implement it in the central laboratory to apply the test in more patients at the same time. The goal is to investigate the added value compared to established renal function parameters in routine diagnostics. To date, our data suggest better and more direct information about kidney function, which may allow for more rapid action. Acute kidney injury is a serious condition that needs to be detected early and counteracted promptly. When the critically ill patients are undergoing renal replacement therapy, the decision to terminate the treatment is of high importance for the success of the therapy. With penKid we may achieve better insights that complement our current practices, and we have strong hopes that this will help us improve patient outcomes on short and long term.” PenKid is a promising biomarker to determine kidney function. It has been studied in over 55,000 patients showing the potential to reflect kidney function in real-time (2,3). Data from the PredARRT-Sep-Trial enrolling patients hospitalized in the surgical ICU of UKHD showed that penKid is a kidney function biomarker with rapid kinetics. According to these findings shared with the scientific community during the AKI&CRRT 2021 conference in San Diego US (4), penKid especially indicates changes in kidney function more dynamically than established functional markers. For those patients in critical conditions who require dialysis, penKid seems to be an early indicator of the kidney function and a potential tool to monitor kidney function and recovery during dialysis. Prof. Dr. med. Markus Weigand, Medical Director of the Clinic for Anesthesiology at UKHD commented “On the ICU we experience the extreme of human disease, and AKI remains a serious complication in critically ill patients. A biomarker alerting changes in the kidney function faster than the currently known standards and uninfluenced by inflammation, has a significant potential for improving patient management. The sooner we identify a patient with kidney dysfunction, the faster we can intervene with life-saving procedures.” Dr. Andreas Bergmann, founder and CSO of SphingoTec added “Testing penKid in routine practice at University Hospital Heidelberg brings the scientific advancements directly to those needing them the most: the patients. AKI is usually diagnosed after symptoms manifest, with diagnostic tests that have limited ability to identify early the onset of the disease. The blind spots in diagnosing kidney dysfunction are well known to the medical community, therefore penKid is a functional biomarker that addresses a clear unmet need and provides actionable information on all stages of the disease evolution.” ## References: 1. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-55. doi: 10.1159/000337487. Epub 2012 Apr 2. PMID: 22473149; PMCID: PMC3362180. 2. Beunders R et al. Proenkephalin Compared to Conventional Methods to Assess Kidney Function in Critically Ill Sepsis Patients. Shock. 2020;54(3):308-14. 3. Donato LJ et al. Analytical performance of an immunoassay to measure proenkephalin. Clin Biochem. 2018;58:72-7 4. https://www.crrtonline.com/conference/support/AKI&CRRT2021_Syllabus.pdf ## About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. Media contact: Ulrike Glaubitz Sr. Public relations specialist SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press@sphingotec.com www.sphingotec.com

• Innovative biomarkers open new avenues for precision medicine approaches in treating acute and critical diseases that need differentiation of the underlying pathophysiology. • Physicians from Europe, Canada, and the USA explore the role of biomarkers in the leap from a “one-fits-all”-treatment to a personalized approach based on the patient’s characteristics. • International medical leaders discuss ways to successfully implement biomarkers penKid, bio-ADM and DPP3 from research into routine clinical practice. Hennigsdorf, Germany, August 8, 2022 – The diagnostics company SphingoTec GmbH (SphingoTec), announces that, during the 4th Scientific Symposium held near Potsdam, Germany, international medical experts discussed the latest developments in diagnostic and therapeutic innovations for advancing precision medicine in critically ill patients. The approach encompasses organ-specific biomarkers working hand in hand with drug candidates and existing therapies to improve the management of diseases such as sepsis and septic shock, acute kidney injury, COVID-19, and cardiogenic shock. The successful transition of the biomarkers from research to clinical routine is an important step that was addressed by current routine users. Biomarkers from research to clinical practice Acute kidney injury (AKI) affects 1 in 3 ICU patients. Since the therapeutical options are limited, the focus lies in managing the symptoms and avoiding the progression of AKI. However, current diagnostics are too late and insensitive, thus resulting in a limited intervention window. Prof. Peter Pickkers, Head of research in Intensive Care Medicine at Radboud University Nijmegen Medical Centre, explained “In AKI we need to bring the diagnostic clock before the current clinical clock to avoid further organ dysfunction. Proenkephalin A 119 - 159 (penKid) is a kidney function biomarker with more swift kinetics that strongly correlates with the actual glomerular filtration rate (GFR). It allows to detect a change in kidney function more than 24 hours earlier compared to the current diagnostic standard creatinine. We have confirmed these findings by developing a formula for estimating the GFR using penKid. After measuring penKid against complex and invasive reference diagnostics and comparing it to existing formulas for kidney function, we have seen a superior performance of the new penKid model.” Notably, the biomarker penKid shows good kinetics in both adults and children (1,3). It is earlier than the current standard of care, and its property of not being influenced by muscle mass, inflammation and common comorbidities allows for monitoring the patient’s condition, especially in critical care (2, 3). Scientists and routine users of penKid highlighted the potential to predict AKI development and recovery, as well as the need for renal replacement therapy (RRT) (3), which are unique features for a kidney biomarker (4, 5). Prof. Gernot Marx, Clinic Director of the University Hospital RWTH Aachen, comments: “We have made the first steps in translating scientific research into clinical practice. Measuring penKid in the morning routine required a change of our procedure and the alignment of all teams. Once established, we have seen immediate benefits in our daily practice that range from better risk stratification to early identification of worsening and improvement of renal function. The potential of this biomarker has not yet been fully exploited, therefore we are currently focused on better understanding the benefits for patients under RRT.” Precision medicine relies on biomarkers for a targeted approach Not only diagnosing but also treating patients correctly can be a major challenge, especially if the underlying mechanisms of a disease vary and require different therapies. This is the case, for example, with sepsis, a disease that encompasses a very heterogeneous group of patients, making the discovery of new therapies very challenging. The biomarkers bioactive adrenomedullin (bio-ADM) and dipeptidyl peptidase 3 (DPP3) distinguish between two independent etiologies of septic shock (6). This facilitates the identification of separate homogenous groups of shock patients with the respective pathological mechanisms. Prof. Alexandre Mebazaa, Head of the Department of Anesthesia and Critical Care Medicine at Lariboisière, Paris stated “The biomarkers bio-ADM and DPP3 are biologically active. This means they can be both, biomarkers that point at different pathophysiologies of sepsis, but also biotargets for new drug candidates that modulate their activity, allowing specific treatment of respective disease mechanisms.” Bio-ADM identifies patients with endothelial dysfunction causing vascular leakage, which escalates into shock and organ dysfunction. The medical experts discussed the latest findings on Adrecizumab, a new promising clinical late-stage drug candidate developed by Adrenomed AG, that targets endothelial barrier dysfunction (7, 8). A separate disease mechanism, leading to hemodynamic instability and shock in sepsis, is the depletion of angiotensin II. DPP3, a biomarker that causes this depletion has been proposed to identify patients who may not be able to develop a positive treatment effect due to the different pathophysiology, thus excluding them from the respective clinical trials. Apart from the use of DPP3 as an enrichment tool for clinical trials, the modulation of DPP3 activity by the antibody Procizumab, a new drug candidate developed by 4TEEN4 Pharmaceuticals, has been discussed (9). Beyond sepsis and septic shock, the identified pathophysiologies could be of relevance in other distinct major mortality drivers such as COVID-19 (10) and cardiogenic shock (11). Dr. Andreas Bergmann, the founder of the three companies SphingoTec, Adrenomed, and 4TEEN4, summarizes: “During the symposium, we have shared the same vision with prestigious representatives of the critical care community: bringing personalized medicine in critical care to the same level the colleagues in oncology have succeeded. At the core of this endeavor are biomarkers that guide clinical decision making and support the development of new therapies. Now SphingoTec’s biomarkers are well positioned for the adoption in clinical routine, laying a strong basis for "precision medicine".” References: 1. Hartman et al, Proenkephalin as a New Biomarker for Pediatric Acute Kidney Injury – Reference Values and Performance in Children Under One Year of Age, Clin Chem Lab Med, 2020; doi: 10.1515/cclm-2020-0381. 2. Kim et al. Proenkephalin Predicts Organ Failure, Renal Replacement Therapy, and Mortality in Patients with Sepsis. Ann Lab Med. 2020;40(6):466-73 3. Caironi P, Latini R, Struck J, Hartmann O, Bergmann A, Bellato V, Ferraris S, Tognoni G, Pesenti A, Gattinoni L, Masson S; ALBIOS Study Investigators. Circulating Proenkephalin, Acute Kidney Injury, and Its Improvement in Patients with Severe Sepsis or Shock. Clin Chem. 2018 Sep;64(9):1361-1369. 4. Khorashadi M, Beunders R, Pickkers P, Legrand M: Proenkephalin: A New Biomarker for Glomerular Filtration Rate and Acute Kidney Injury. Nephron 2020;144:655-661. Doi: 10.1159/000509352 5. Hollinger et al, Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study, Kidney Int Rep, 2018; doi: 10.1016/j.ekir.2018.08.006 6. van Lier (2020), Promotion of vascular integrity in sepsis through modulation of bioactive adrenomedullin and dipeptidyl peptidase 3, J. Intern. Med., DOI: doi.org/10.1111/joim.13220 7. Laterre PF, et al. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: the AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. 2021 Nov;47(11):1284-1294. Doi: 10.1007/s00134-021-06537-5. 8. Geven et al, Effects of the Humanized Anti-Adrenomedullin Antibody Adrecizumab (HAM8101) on Vascular Barrier Function and Survival in Rodent Models of Systemic Inflammation and Sepsis. Shock. 2018 Dec;50(6):648-654. Doi: 10.1097/SHK.0000000000001102. 9. Deniau et al, Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur J Heart Fail, 2020; 22: 290-299. Doi: 10.1002/ejhf.1601 10. Clinicaltrials.gov/ct2/show/NCT05156671 11. Takagi K, Blet A, Levy B, Deniau B, Azibani F, Feliot E, Bergmann A, Santos K, Hartmann O, Gayat E, Mebazaa A, Kimmoun A. Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: results from the OptimaCC trial. Eur J Heart Fail. 2020 Feb;22(2):279-286. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction, and monitoring of critical medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis, and Proenkephalin (penKid), a biomarker for real-time assessment of kidney function. Dipeptidyl Peptidase 3 (DPP3) is a biomarker for cardiac depression, in-licensed from 4TEEN4 Pharmaceuticals GmbH (www.4teen4.de). IVD tests for SphingoTec’s biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA). The Nexus IB10 portfolio is complemented by established and commonly used biomarker tests for acute and critical care such as PCT, Troponin, NT-proBNP, D-Dimer, TSH and others. Media contact: Dr. Ulrike Glaubitz Senior Public Relations Specialist SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-113

• Bio-ADM is a biomarker for the assessment of endothelial dysfunction, a hallmark of sepsis, and has been widely studied in intensive care units (ICU) • Clinical trial in the emergency department (ED) shows that bio-ADM is predictive of ICU admission and may be used for early stratification of unselected sepsis patients • The published data show that high levels of bio-ADM are predicting the risk for multiple organ failure and mortality, while low levels are associated with ED discharge Hennigsdorf, Germany, August 1, 2022 – The diagnostics company SphingoTec GmbH (SphingoTec) announces new findings that support the clinical value of the biomarker bio-ADM for triaging sepsis patients in ED, exceeding the prognostic performance of routine biomarkers. So far, the predictive potential of the innovative biomarker has been confirmed and used primarily in ICU. A new study, recently published, confirms its applicability in ED (1). A severe infection can quickly progress to sepsis - a life-threatening situation. Unfortunately, sepsis is difficult to diagnose and treat because it has a high range of forms and intensities. However, successful treatment is primarily time-dependent (2): an early diagnosis can save lives! In ED, it is still difficult to identify sepsis patients early, and assigning the correct further treatment is thus challenging. Adrenomedullin, a hormone that has homeostatic and regulatory effects on various body systems (3,4), as well as playing a role in hemodynamic regulation, and is measurable via the biomarker bioactive Adrenomedullin (bio-ADM), may help to bridge this gap. Biomarkers can provide information about the underlying causes of a disease and allow predictions about potential changes in patient condition in the next hours to days. Bio-ADM is an already known biomarker in intensive care units (5). Now, it has been tested in ED whether there was an association between bio-ADM and sepsis severity. Furthermore, it was examined whether bio-ADM would allow distinguishing between patients who could be discharged from ED from those who, for example, are at high risk for multiple organ failure due to sepsis and thus need to be transferred to the ICU. Prof. Olle Melander, Professor and Senior Physician, Lund University and Skåne University Hospital in Sweden, explains: "The results of our study suggest that bio-ADM is potentially an important clinical biomarker in ED and could be used to allow early triage of sepsis patients. We demonstrated that bio-ADM allows a significantly better prediction of the sepsis severity than other biomarkers. Accordingly, high bio-ADM levels in ED are associated with mortality, as well as the development of severe multiple organ failure, while low levels indicate that patients may be discharged”. Dr. Andreas Bergmann, founder, and CSO of SphingoTec adds: "In ED, triage is of enormous importance to provide patients with the right treatment. The study results indicate that previous experiences with the biomarker bio-ADM in ICU could be transferred to ED. The ability of bio-ADM to identify high-risk patients thus could help ED physicians to optimize their decision-making and patient management." References: 1. Lundberg OHM, Rosenqvist M, Bronton K, Schulte J, Friberg H, Melander O (2022) Bioactive adrenomedullin in sepsis patients in the emergency department is associated with mortality, organ failure, and admission to intensive care. PLoS ONE 17(4): e0267497. https://doi.org/10.1371/journal.pone.0267497 2. World Health Organization Improving the prevention, diagnosis and clinical management of sepsis 2017, April 13. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_13-en.pdf?ua=1 3. Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993; 192(2):553–60. https://doi.org/10.1006/bbrc.1993.1451 4. Samson WK, Resch ZT, Murphy TC, Vargas TT, Schell DA. Adrenomedullin: Is There Physiological Relevance in the Pathology and Pharmacology? News Physiol Sci. 1999; 14:255–9, https://doi.org/10.1152/physiologyonline.1999.14.6.255 5. Lundberg OHM, Lengquist M, Spangfors M, Annborn M, Bergmann D, Schulte J, et al. Circulating bioactive adrenomedullin as a marker of sepsis, septic shock, and critical illness. Crit Care. 2020; 24 (1):636. https://doi.org/10.1186/s13054-020-03351-1 About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis, and Proenkephalin (penKid), a biomarker for real-time assessment of kidney function. Dipeptidyl Peptidase 3 (DPP3), a biomarker for cardiac depression. IVD tests for SphingoTec’s biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA). The Nexus IB10 portfolio is complemented by established and commonly used biomarker tests for acute and critical care such as PCT, Troponin, NT-proBNP, D-Dimer, TSH and others. Media contact: Dr. Ulrike Glaubitz Senior Public Relations Specialist SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-113

• SphingoTec appoints Jörg Menten as Chief Executive Officer (CEO) as from July 1, 2022 to drive the commercialization and scale its diagnostic business. • SphingoTec is a commercial-stage diagnostic company focusing on innovative critical care diagnostics made available on multiple IVD platforms. • Founder Dr. Andreas Bergmann transitions his role as CEO to Chief Scientific Officer (CSO) to ensure scientific leadership of the company. Hennigsdorf/Berlin, Germany, June 30, 2021 – Diagnostics company SphingoTec GmbH (“SphingoTec”) today announced that Jörg Menten was appointed CEO of SphingoTec effective of July 1, 2022. Jörg Menten, previously acting as Chief Commercial Officer of the company, will focus on successfully commercializing the biomarker-based diagnostics that address high unmet needs in critical care settings. Dr. Andreas Bergmann assumes the role of CSO within SphingoTec to further develop strategic innovations. Jörg Menten is a highly skilled senior executive with a long-standing track record of leadership positions in the global healthcare industry. With an MBA from the University of Mannheim, he spent more than 13 years with Boehringer Mannheim in several roles contributing to its steep global growth in all care settings – patient monitoring, Point of Care, and Lab Diagnostics. As CFO of the Boehringer Mannheim Group, he had a significant role in its acquisition through Roche AG in 1998. Menten served as President International for Kinetic Concepts Inc. driving the global adoption of its wound-healing technology VAC® and its IPO on NYSE in 2004. Later, he served as CEO of Vanguard AG, Berlin, a European market leader in hospital services. In his position as President International for CeloNova Inc. he led the global commercialization of Embozene Tandem®, an innovative drug delivery system applied in Interventional Radiology settings for the treatment of liver cancer; acquired by Boston Scientific in 2015. Jörg Menten commented on his appointment, “I am pleased to support SphingoTec in such an exciting time in its evolution. The products developed under the leadership of Dr. Andreas Bergmann have the potential to change the standard of care in diagnosing critically ill patients. Together with Dr. Angelo Moesslang, Chief Financial Officer, we have the clear mission of bringing innovations to the patients. I look forward to further scaling and accelerating SphingoTec’s growth.” Gerald Möller, Chairman of the Advisory Board at SphingoTec, said, „On behalf of the Advisory board and all the employees, I would like to thank Dr. Andreas Bergmann for his restless dedication to discovering actionable Biomarkers in Critical Care. These are the basis for developing precision medicine from research to medical practice in a global business. The successful integration of our Nexus POC platform advanced SphingoTec to a diagnostic solution provider. With his future support as CSO, we can continue the innovation legacy at SphingoTec. On behalf of the Advisory Board, I welcome Jörg Menten in his new capacity as CEO of SphingoTec. His tremendous experience in our global industry will help grow our company to become a key provider of diagnostic innovations in Critical Care. Dynamics and discipline will foster the company’s expansion. His experience as CCO of SphingoTec will help drive and accelerate growth and success. I look forward to a creative and exciting dialogue with Jörg and the Management Team.” Dr. Andreas Bergmann explained, “SphingoTec has now reached a full commercial stage, and we have built a strong leadership team that can support scaling the business. As my personal goal lies in further developing medical innovations, I have decided to shift my focus at SphingoTec from the CEO role to the CSO position. With this change, I will be able to direct my resources on the further development and research of the products at SphingoTec and on developing precision medicine solutions.” About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction, and monitoring of critical medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis, and Proenkephalin (penKid), a biomarker for real-time assessment of kidney function. Dipeptidyl Peptidase 3 (DPP3) is a biomarker for cardiac depression. IVD tests for SphingoTec’s biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA). The Nexus IB10 portfolio is complemented by established and commonly used biomarker tests for acute and critical care such as PCT, Troponin, NT-proBNP, D-Dimer, TSH and others. Media contact: Dr. Ulrike Glaubitz Senior Public Relations Specialist SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-113

• A multi-center study led by UKE is evaluating one of the first personalized approaches for moderate to severe COVID-19 patients • The innovative biomarkers bio-ADM and DPP3 are guiding the enrollment of patients suffering from endothelial barrier dysfunction • The biomarkers are measured with SphingoTec’s point of care tests for timely decision making. Hennigsdorf/Berlin, Germany, April 11, 2022 - Diagnostics company SphingoTec GmbH (SphingoTec) announced the implementation of two critical care biomarkers to guide the application of a potential new drug candidate for the treatment of COVID-19 patients. The personalized approach will allow to distinguish between separate clinical pathways, requiring different treatment decisions. This trial will implement the biomarker bioactive Adrenomedullin (bio-ADM) for early identification of endothelial dysfunction (1), which plays a central role in the pathophysiology of COVID-19 (2). The drug candidate Adrecizumab targets septic shock patients with high bio-ADM levels to restore the endothelial barrier function (3), and has already shown favorable outcomes in a case series of eight severe COVID-19 patients (4). At the same time, patients with high Dipeptidyl peptidase 3 (DPP3) levels will not be included in the trial. The biomarker DPP3 is indicating another underlying disease mechanism leading to cardiac depression and requiring different therapeutic options (5). Both critical care biomarkers are measured via SphingoTec’s rapid point of care platform Nexus IB10. For more information visit https://sphingotec.com/science/acute-care-pathways/covid-19 . PD Dr. med. Mahir Karakas (Department of Intensive Care Medicine at UKE) said, “Not all COVID-19 patients have the same disease course and the pathology is very different. Therefore, we have to adapt the treatment to each individual patient. We will now use in a double blind randomized multicenter trial the biomarker bio-ADM and DPP3 which proved to be useful for patient enrichment in a phase II trial for treatment of septic shock.” This is one of the first personalized, biomarker-guided trials in moderately to severely ill COVID-19 patients. The multicenter study led by UKE plans to enroll more than 200 patients for treatment with the new compound (6). Adrecizumab is provided by Adrenomed AG (www.adrenomed.com), and the critical care biomarkers are determined via SphingoTec’s rapid point of care platform Nexus IB10. Dr. Andreas Bergmann, founder of SphingoTec and co-founder of Adrenomed stated: “The innovative biomarkers generate better insights into the pathophysiology of critically ill patients. This is translated into clear decisions that allow a better treatment guidance and ultimately reduce mortality. This study represents a leap from “one drug fits all” to a personalized approach in treating COVID-19.” References: (1) Simon T-P et al. Prognostic Value of Bioactive Adrenomedullin in Critically Ill Patients with COVID-19 in Germany: An Observational Cohort Study. Journal of Clinical Medicine. 2021, 10, 1667. doi.org/10.3390/jcm10081667 (2) Varga et al. Endothelial cell infection and endothelitis in COVID-19. The Lancet, 2020, DOI: https://doi.org/10.1016/S0140-6736(20)30937-5 (3) Laterre PF, et al. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: the AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. 2021 Nov;47(11):1284-1294. doi: 10.1007/s00134-021-06537-5. (4) Karakas, M et al. Targeting Endothelial Dysfunction in Eight Extreme-Critically Ill Patients with COVID-19 Using the Anti-Adrenomedullin Antibody Adrecizumab (HAM8101). Biomolecules 2020, 10, 1171. https://doi.org/10.3390/biom10081171 (5) van Lier et al 2020, Promotion of vascular integrity in sepsis through modulation of bioactive adrenomedullin and dipeptidyl peptidase 3, J. Intern. Med., DOI: doi.org/10.1111/joim.13220 (6) https://clinicaltrials.gov/ct2/show/NCT05156671 ### About SphingoTec SphingoTec GmbH develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis or COVID-19, Proenkephalin (penKid), a biomarker for real-time assessment of kidney function. Dipeptidyl Peptidase 3 (DPP3), a biomarker for cardiac depression was in-licensed from 4TEEN4 Pharmaceuticals GmbH (www.4teen4.de). IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA). The Nexus IB10 portfolio is complemented by established and commonly used biomarker tests for acute and critical care such as PCT, Troponin, NT-proBNP, D-Dimer, TSH and others. Find out more www.sphingotec.com Media contact: Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press@sphingotec.com www.sphingotec.com
• Post-surgical increase of endothelial function biomarker bioactive adrenomedullin (bio-ADM) concentrations predicts organ dysfunction. • Bio-ADM may help identify patients with an increased risk of developing prolonged vasopressor dependence and prolonged ICU stay after cardiac surgery. • Increased blood concentrations of cardiac depressant factor dipeptidyl peptidase 3 (DPP3) are related to the complexity and duration of the intervention. Hennigsdorf, Germany, March 24, 2022 - The diagnostic company SphingoTec GmbH (SphingoTec) announces new evidence supporting the use of innovative biomarkers in the early identification of organ dysfunction and risk stratification of cardiac surgery patients (1). Despite many serious post-operative complications (2), there are currently limited tools available for the early identification of organ dysfunction in these patients (3,4). Scientists from the Radboud University Medical Center have analyzed the predictive value of bio-ADM and DPP3 for short-term outcomes in a prospective study in cardiac surgery patients. Bio-ADM and DPP3 represent two distinct molecular pathways involved in the development of circulatory shock (5). Compromised hemodynamics after surgery triggers the release of the hormone bio-ADM, a key regulator of endothelial barrier function and vascular tone. The current data shows that bio-ADM at day two after ICU admission effectively differentiated between high-risk and low-risk patients for developing organ failure. The study data also confirmed the independency of a second pathophysiological process, the release of DPP3 upon cell death. Surgery acts as an additional factor leading to an increase in DPP3 in the bloodstream, which is mainly related to tissue injury and the extent of the procedure. Therefore, elevated levels of DPP3 decrease shortly after the surgery. Not surgery-induced and persistently high concentration of DPP3 signals impaired tissue perfusion and high risk for organ failure (5). Dr. Andreas Bergmann, founder and CEO of SphingoTec, added, “Cardiac surgery is often accompanied by postoperative complications. By using the innovative biomarkers, clinicians can improve the risk stratification provided by conventional risk scores and improve their patient management.” References: 1. van Lier et al. The value of bioactive adrenomedullin and dipeptidyl peptidase 3 to predict short-term unfavourable outcomes after cardiac surgery: A prospective cohort study. Eur J Anaesthesiol. 2022 Apr 1;39(4):342-351. doi: 10.1097/EJA.0000000000001662. 2. Nearman et al. Perioperative complications of cardiac surgery and postoperative care. Crit Care Clin 2014; 30:527–555. 3. Paparella et al. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 2002; 21:232–244. 4. Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology 2002; 97:215–252 5. van Lier D, Kox M, Pickkers P. Promotion of vascular integrity in sepsis through modulation of bioactive adrenomedullin and dipeptidyl peptidase 3. J Intern Med 2020; 289:792–806. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis, and Proenkephalin (penKid), a biomarker for real-time assessment of kidney function. Dipeptidyl Peptidase 3 (DPP3), a biomarker for cardiac depression. IVD tests for SphingoTec’s biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA). The Nexus IB10 portfolio is complemented by established and commonly used biomarker tests for acute and critical care such as PCT, Troponin, NT-proBNP, D-Dimer, TSH, and others. Media contact SphingoTec: Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0

• The collaboration focuses on SphingoTec’s near patient diagnostic solution for critical care settings. • Rivaara gains exclusive rights to market and distribute the Nexus IB10 platform and its rapid tests for innovative biomarkers and commonly used parameters in the Indian subcontinent. • The Nexus IB10 technology is a “hand-held laboratory” providing fast and reliable diagnostics to support the management of critically ill patients. Hennigsdorf, Germany and Mumbai, India, March 17, 2022 - The diagnostic company SphingoTec GmbH (SphingoTec) and Rivaara Labs Pvt Ltd. (Rivaara) today announced that they have entered into a multi-year distribution agreement for the commercialization of SphingoTec’s point of care diagnostic solutions in the Indian subcontinent. The Nexus IB10 analyzer and its portfolio of rapid tests allow convenient assessment of biomarkers relevant in critical care conditions such as sepsis, acute kidney injury, cardiogenic shock, acute heart failure, and myocardial infarction. Under the terms of the agreement, Rivaara will have the exclusive right to market and distribute SphingoTec’s point of care tests for innovative biomarkers in the Indian subcontinent. This includes the biomarkers proenkephalin (penKid) for the assessment of kidney function (1), bioactive adrenomedullin (bio-ADM) for the assessment of endothelial function (2), and dipeptidyl peptidase 3 (DPP3) a biomarker for cardiac depression (3). The innovative markers are complemented by commonly used parameters such as procalcitonin, troponin, D-dimer, NT-proBNP, and TSH. The biomarkers are made available as rapid tests on the Nexus IB10 bedside technology, a fully automated immunoassay point-of-care instrument, which provides accurate test results within only 20 minutes from whole blood samples. The Nexus IB10 analyzer and its test portfolio can be flexibly deployed in a variety of point of care settings, including emergency departments, intensive care units, and laboratories. Dr. Angelo Moesslang, Managing Director and Chief Financial Officer of SphingoTec, said, “This distribution agreement is an important step in making our near-patient diagnostics globally available. A point of care solution for acute and critical cases can enable healthcare organizations to maximize diagnostic resources and help clinicians make more informed decisions.” Mr. Shyamakant Giri, Chief Executive Officer of Rivaara said, “Adding the point of care platform and its rapid tests to our portfolio is increasing the settings in which we facilitate integrated diagnostic testing in the patient-care pathway. Not only are we able to provide well established tests used on a daily basis, but we can also enable access to innovative biomarkers, which address unmet needs in disease areas such as acute kidney injury and sepsis.” To discover more about the Nexus IB10 and SphingoTec’s portfolio of diagnostic solutions for acute and critical care, please visit www.sphingotec.com. For more information on the Rivaara Labs portfolio, please visit www.rivaaralabs.com References: 1. Beunders et. al. Proenkephalin (PENK) as a Novel Biomarker for Kidney Function, The Journal of Applied Laboratory Medicine, Volume 2, Issue 3, 1 November 2017, Pages 400–412, doi: https://doi.org/10.1373/jalm.2017.023598 2. Geven et. al. Vascular Effects of Adrenomedullin and the Anti-Adrenomedullin Antibody Adrecizumab in Sepsis, SHOCK: August 2018 - Volume 50 - Issue 2 - p 132-140 doi: 10.1097/SHK.0000000000001103 3. Boorsma et. al. Dipeptidyl peptidase 3, a marker of the antagonist pathway of the renin-angiotensin-aldosterone system in patients with heart failure. Eur J Heart Fail. 2021 Jun;23(6):947-953. doi: 10.1002/ejhf.2158. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis, and Proenkephalin (penKid), a biomarker for real-time assessment of kidney function. Dipeptidyl Peptidase 3 (DPP3), a biomarker for cardiac depression was in-licensed from 4TEEN4 Pharmaceuticals GmbH (www.4teen4.de). IVD tests for SphingoTec’s biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA). The Nexus IB10 portfolio is complemented by established and commonly used biomarker tests for acute and critical care such as PCT, Troponin, NT-proBNP, D-Dimer, TSH and others. About Rivaara Labs Rivaara Labs Pvt Ltd. (“Rivaara”, Mumbai, India) is harnessing the power of Molecular diagnostics to bring disruptive, affordable, and innovative need-based diagnostic solutions to India. With an extensive range of test services, Rivaara offers sample-to-answer qualified solutions based on a broad portfolio of automated RT-PCR, IT connectivity, superior analysis, and LIMS interface with actionable clinical reports across infectious diseases, oncology, antibiotic resistance, pathogen detection, inherited disease testing and precision medicine. The solutions address a significant unmet medical need for timely, actionable information that addresses the challenges of the current standard of care for diagnosing and monitoring the diseases. The aim is to develop a strong network of specialized Molecular Diagnostics labs, market novel point of care solutions and build a best-in-class national reference lab. Apart from the investment in infrastructure, Rivaara also invests in highly inspired and motivated human capital within the organization. Media contact SphingoTec: Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf

• For children burdened with life-threatening acute kidney injury (AKI) there are no reliable diagnostics to assist the management of pediatric AKI. • The biomarker proenkephalin (penKid) sets a firm foundation in pediatric kidney assessment as it can identify fast and accurate pediatric AKI and its severity, overcoming current limitations. Hennigsdorf, Germany, March 15, 2022 - The diagnostic company SphingoTec GmbH (SphingoTec) SphingoTec GmbH (SphingoTec) announces that its kidney function biomarker penKid is reliably identifying AKI in infants, offering a solution to the shortcomings in pediatric critical care. AKI is mostly diagnosed too late for successful intervention which leads to steep mortality rates and chronic kidney disease (1,2). AKI is a life-threatening complication to every 1 in 3 children in intensive care - a shockingly high prevalence (1). The current routine measurements are not only biased by non-AKI related factors (3), but also identify with an unacceptable delay of 72 hours the impairment, when 50% of the kidney function is already lost (4). PenKid is a blood-based biomarker that supports clinicians overcoming these limitations. High or increasing penKid concentrations accurately predict AKI and its severity in infants (5), offering an unprecedented alternative to managing pediatric AKI. PenKid is not influenced by non-renal factors making it easy to adopt in critical care (6,7). As a non-invasive biomarker, penKid provides unparalleled real-time and accurate information for the early recognition, severity, and recovery from AKI (8,9). Since AKI is a sudden and severe disease, this information can help vital interventions and improve recovery. Dr. Andreas Bergmann, CEO of SphingoTec, commented: “AKI is a challenging setting in pediatric critical care with persisting unmet diagnostic needs. With clinical symptoms showing only shortly before kidney failure, it remains a blind spot and a silent threat on the pediatric wards. PenKid offers a solution for the early recognition of AKI in children which is a main condition for improving their prognosis.” During the recent scientific sessions at AKI & CRRT 2022 in San Diego, CA, Prof. Peter Pickkers (Radboud University, Nijmegen) presented the data on penKid in pediatric AKI, alongside a new formula that translates penKid into a familiar scale for the clinical routine use initially in adults. Data from the researchers of Radboud University, The Netherlands, and Mayo Clinic, US confirms that the new formula is overcoming the limitations of the current clinical practice. References: 1. Sutherland et al (2015) AKI in Hospitalized Children: Comparing the pRIFLE, AKIN, and KDIGO Definitions, Clin J Am Soc Nephrol, doi: 10.2215/CJN.01900214. 2. Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med 2017;376:11-20. 3. Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr 2012;24:191-6. 4. Bennett MR, Nehus E, Haffner C, Ma Q, Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr Nephrol 2015;30:677-85. 5. Hartman et al (2020), Proenkephalin as a New Biomarker for Pediatric Acute Kidney Injury - Reference Values and Performance in Children Under One Year of Age, Clin Chem Lab Med, doi: 10.1515/cclm-2020-0381. 6. Caironi et al. Circulating Proenkephalin, Acute Kidney Injury, and Its Improvement in Patients with Severe Sepsis or Shock. Clin Chem. 2018;64(9):1361-9. 7. Kim et al. Proenkephalin Predicts Organ Failure, Renal Replacement Therapy, and Mortality in Patients with Sepsis. Ann Lab Med. 2020;40(6):466-73 8. Marino et al (2015), Diagnostic and short-term prognostic utility of plasma proenkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department, J Nephrol, DOI 10.1007/s40620-014-0163-z 9. Hollinger et at (2018), Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study, Kidney Int Rep, DOI: 10.1016/j.ekir.2018.08.006 About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis, and Proenkephalin (penKid), a biomarker for real-time assessment of kidney function. Dipeptidyl Peptidase 3 (DPP3), a biomarker for cardiac depression. IVD tests for SphingoTec’s biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA). The Nexus IB10 portfolio is complemented by established and commonly used biomarker tests for acute and critical care such as PCT, Troponin, NT-proBNP, D-Dimer, TSH and others. Media contact SphingoTec: Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0

SphingoTec expands leadership team to facilitate scaling of its fast-growing diagnostics business. The company commercializes innovative biomarkers for critical care settings and makes them available on multiple IVD instrument platforms. Jörg Menten will lead the company’s commercial efforts and drive expansion into global markets. Hennigsdorf/Berlin, Germany, November 29, 2021 – Diagnostics company SphingoTec GmbH (“SphingoTec”) today announced the appointment of Jörg Menten as its Chief Commercial Officer. In this role, Menten will develop the global commercial strategy, oversee the expansion in diagnostic markets worldwide, strengthen existing customer relations and forge new partnerships with global IVD leaders and pharmaceutical companies. “We are committed to bringing personalized medicine to critical care,” said Dr. Andreas Bergmann, Chief Executive Officer and founder of SphingoTec. “I welcome Mr. Menten on board. His wealth of experience in the healthcare industry provides SphingoTec with strong commercial leadership in accelerating the adoption of our novel biomarkers in the clinical routine. Our goal is to improve patient management with evidence-driven treatment decisions.” SphingoTec focuses on discovering, developing, and commercializing novel, biomarker-based diagnostics for personalized medicine in critical care settings. The innovative diagnostic solutions are made available on the proprietary point of care platform Nexus IB10 and laboratory diagnostic platforms. SphingoTec’s bedside technology is complemented by a broad portfolio of standard parameters, making it an ideal addition to hospitals, laboratories, and other near-patient settings. Menten will lead the team to build new partnerships for the out-licensing of biomarkers to global diagnostics companies as well as drive companion diagnostics partnerships with the pharmaceutical industry. Jörg Menten is a highly skilled senior executive with a long-standing track record of leadership positions in the global healthcare industry. With an MBA from the University of Mannheim, he spent more than 13 years with Boehringer Mannheim in several roles contributing to its steep global growth in all care settings – patient monitoring, Point of Care and Lab Diagnostics. As CFO of the Boehringer Mannheim Group, he had a significant role in its acquisition through Roche AG in 1998. Menten served as President International for Kinetic Concepts Inc. driving the global adoption of its wound healing technology VAC® and its IPO on NYSE in 2004. Later, he served as CEO of Vanguard AG, Berlin, a European market leader in hospital services and as President International for CeloNova Inc. leading the global commercialization of Embozene Tandem®, an innovative drug delivery system applied in Interventional Radiology settings for the treatment of liver cancer; acquired by Boston Scientific in 2015. Jörg Menten commented, “SphingoTec has all assets in place for successful commercialization! SphingoTec’s biomarkers are covering main unmet medical needs in critical care: real-time kidney function assessment, real-time endothelial function assessment, cardiac depression, diagnosis of sepsis, diagnosis of acute heart failure, diagnosis of myocardial infarction, and diagnosis of lung embolism. I am excited to join this team and to lead the market development and commercialization of our biomarkers and POC platform.” About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA). The Nexus IB10 portfolio is complemented by established and commonly used biomarker tests for acute and critical care such as PCT, Troponin, NT-proBNP, D-Dimer, TSH and others. Media contact: Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press[at]sphingotec.com www.sphingotec.com

• Sepsis is a life-threatening medical emergency that quickly escalates into multiple organ failure. • SphingoTec’s biomarkers for assessing organ function in real-time help guide clinical decision making by providing patient-specific information. • Multiple presentations at the Weimar Sepsis Update in Weimar help push forward the science and practice of precision medicine with diagnosis and biomarker-guided therapy. Hennigsdorf/Berlin, Germany, September 9, 2021 – Diagnostics company SphingoTec GmbH (“SphingoTec”) announces a series of presentations demonstrating the utility of its innovative biomarkers in sepsis, positively impacting diagnostic and treatment decisions for critically ill patients. The new data will be presented at the 10th Weimar Sepsis Update, Germany. In the oral presentation on the “Properties of Proenkephalin in septic acute kidney injury”, Dr. med. Christian Nusshag presented the results of a prospective observational study and the performance of kidney function biomarker Proenkephalin (penKid). Measured on top of standard of care, penKid can reliably indicate changes in renal function more dynamically than serum creatinine and can also provide additional diagnostic information on renal function, even when the patient is under renal replacement therapy. In addition to the research presentation, during a lunch symposium, new data will highlight the utility of the biomarkers in clinical routine as well as in biomarker-guided clinical trials in sepsis. In the presentation “Implementing new Biomarkers in Daily Practice: Proenkephalin and bioactive Adrenomedullin in sepsis”, Dr. med. Lukas Martin from the Uniklinik RWTH Aachen, shares the insights from the first hospital to have implemented the innovative biomarkers in clinical routine. The regular measurements of the biomarkers penKid and bio-ADM have supported clinicians to improve diagnosis, help earlier risk stratification and optimize the treatment of critically ill patients. Bioactive adrenomedullin (bio-ADM) is a dynamic biomarker delivering real-time information on the endothelial function, one of the most common causes of septic shock. Moreover, increased bio-ADM levels were used as inclusion criteria in the AdrenOSS-2 Phase II trial to identify patients with endothelial dysfunction. In the presentation “Biomarker-guided Approach for the Treatment of Septic Shock – Precision Medicine in the AdrenOSS-2”, Prof. Dr. med. Tobias Schürholz from the Uniklinik RWTH Aachen will show a closer look at the steps taken to achieve precision medicine in septic organ failure. Dr. Andreas Bergmann, CEO and founder of SphingoTec said “it is clear that sepsis is not only characterized by a high incidence worldwide, but it is also diagnostically underserved. Our biomarkers provide actionable information on pathophysiological mechanisms in sepsis. By early identifying and monitoring the hallmarks of sepsis, such as endothelial dysfunction and kidney dysfunction, our biomarkers can provide patient-specific information, paving the way for personalized medicine in sepsis, and beyond.” ## 10th Weimar Sepsis Update Oral presentation • Properties of Proenkephalin in septic acute kidney injury. Speaker: Dr. Med. Christian Nusshag, University Hospital Heidelberg. Session: WS2: European Group on Immunology of Sepsis (EGIS) Lunch symposium: Precision Medicine in Septic Organ Failure • Implementing new Biomarkers in Daily Practice: Proenkephalin and bioactive Adrenomedullin in sepsis. Speaker: Priv. Doz. Dr. Med. Lukas Martin, Uniklinik RWTH Aachen • Biomarker-guided Approach for the Treatment of Septic Shock – Precision Medicine in the AdrenOSS-2. Speaker: Prof. Dr. Med. Tobias Schürholz, Uniklinik RWTH Aachen ## About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform. SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) produces the tests alongside a broad menu of established and commonly used tests for acute and critical care. Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press@sphingotec.com www.sphingotec.com
Endothelial and kidney dysfunction are common complications in septic patients leading to organ failure and unfavorable outcomes. The organ-specific biomarkers bio-ADM and penKid are used in clinical routine for monitoring the endothelium and respectively kidney function in intensive care units. Prof. Dr. Gernot Marx of Uniklinik RWTH Aachen is presenting at ISICEM new evidence on how the use of biomarkers can support clinicians in the management of sepsis. Aachen, Germany and Hennigsdorf/Berlin, Germany, August 31, 2021 – German University Hospital Uniklinik RWTH Aachen (“Uniklinik RWTH Aachen”) and diagnostics company SphingoTec GmbH (“SphingoTec”) announce today that novel data on the acute care biomarkers bioactive adrenomedullin (bio-ADM) and proenkephalin (penKid) will be presented at the 40th International Symposium on Intensive Care and Emergency Medicine (ISICEM) taking place in Brussels, Belgium on August 31 - September 3, 2021. Sepsis is a medical emergency in need of quick therapeutic decisions, although it often progresses undetected in its early stages and remains difficult to manage in the late stages. The organ specific biomarkers first introduced in the clinical routine at Uniklinik RWTH Aachen, Germany offer a new perspective on the disease pathology and facilitate a more timely and efficient treatment. Prof. Dr. Gernot Marx, the Director of the Clinic for Operative Intensive Care and Intermediate Care at Uniklinik RWTH Aachen, one of the largest ICU wards in Europe, explains “Septic patients are at high risk of developing life-threatening complications. The use of biomarkers that can facilitate a faster and better diagnosis and monitoring of sepsis progression allows clinicians to provide the best available treatment. I am excited to share with the critical care community the latest insights on improving septic patients’ management with the help of innovative biomarkers.” PenKid is a biomarker for the real-time assessment of kidney function. It is a blood-based solution for detecting true glomerular filtration rate (true GFR) in clinical routine testing and is independent from inflammation and comorbidities (1). PenKid has been proven to not only predict septic acute kidney injury earlier than today’s standard of care, but also to detect the presence and severity of the disease, identify patients at high risk of unfavorable outcomes and indicate the renal recovery (1,2). Bio-ADM is a biomarker for the real-time assessment of endothelial function (3). The endothelium is the interior wall of the blood vessels that acts as a barrier separating the blood from its surroundings. Bio-ADM enables the assessment of endothelial function up to 48 hours before the symptoms become visible. High bio-ADM levels indicate severe hypotension, edema formation, need for immediate therapeutic interventions and for organ support. Being a dynamic biomarker, low or decreasing bio-ADM blood levels indicate improved outcomes (3,4). ### 40th ISICEM Presentation: Session: Sepsis Biomarkers Title: “Endothelial and kidney function biomarkers to guide management” Speaker: Dr. Gernot Marx, Uniklinik RWTH Aachen, Germany Date & Time: September 1. at 5:15 p.m. CET ### References (1) Khorashadi M, et al. Proenkephalin: A New Biomarker for Glomerular Filtration Rate and Acute Kidney Injury. Nephron. 2020;144(12):655-61 DOI: 10.1159/000509352 (2) Hollinger A, et al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int Rep. 2018;3(6):1424-33. DOI: 10.1016/j.ekir.2018.08.006 (3) Geven C, et al. Vascular Effects of Adrenomedullin and the Anti-Adrenomedullin Antibody Adrecizumab in Sepsis. Shock. 2018 Aug;50(2):132-140. doi: 10.1097/SHK.0000000000001103. (4) van Lier D, et al. Promotion of vascular integrity in sepsis through modulation of bioactive adrenomedullin and dipeptidyl peptidase 3. J Intern Med. 2021 Jun;289(6):792-806. doi: 10.1111/joim.13220. Uniklinik RWTH Aachen The Uniklinik RWTH Aachen is a supramaximal care provider that combines patient-oriented medicine and nursing, teaching and research at an international level. With 36 specialist clinics, 28 institutes and five interdisciplinary units, the University Hospital covers the entire medical spectrum. Excellently qualified teams of doctors, nurses and scientists are competently committed to the health of the patients. The bundling of patient care, research and teaching in one central building offers the best conditions for intensive interdisciplinary exchange and close clinical and scientific networking. Around 8.000 employees provide patient-oriented medicine and care according to recognized quality standards. With 1.400 beds, the University Hospital treats around 50.000 inpatient and 200.000 outpatient cases per year. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform. SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) produces the tests alongside a broad menu of established and commonly used tests for acute and critical care. Press contact Dr. Mathias Brandstädter Leitung Unternehmenskommunikation Uniklinik RWTH Aachen Pauwelsstraße 30 52074 Aachen Telefon: 0241 80-89893 mbrandstaedter@ukaachen.de www.ukaachen.de Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press@sphingotec.com www.sphingotec.com

Dr. Möller’s appointment will strengthen SphingoTec capacities to drive global expansion SphingoTec develops biomarker tests for critical care settings and markets them on its point of care analyzer, Nexus IB10. Hennigsdorf/Berlin, Germany, August 23, 2021 – Diagnostics company SphingoTec GmbH (“SphingoTec”) announces that Dr. Gerald Möller has joined the company’s advisory board as chairman of the board. With over 40 years of experience in diagnostics and life sciences, Dr. Möller will guide the company’s strategic expansion. SphingoTec is a fully integrated diagnostic company that develops innovative biomarkers for critical care settings and makes them available as rapid tests on its point of care analyzer. Dr. Andreas Bergmann, CEO and founder of SphingoTec said, “We are very pleased to welcome Dr. Möller as chairman of our advisory board. Dr. Möller brings a wealth of knowledge and expertise accumulated throughout a distinguished international career. His perspective and experience will be an invaluable asset as we head into the next phase of our development.” Dr. Möller takes over the chair of the advisory board from Dr. Ute Kilger who will continue leveraging her skills and experience as member of the advisory board. “I am confident that our combined experience in key areas will play a valuable role in guiding strategic decisions and broadening SphingoTec’s global footprint,” added Dr. Kilger. Commenting on the appointment, Dr. Gerald Möller said, “I am excited about the opportunity to serve the company now as Chairman of the Advisory Board. The next phase of SphingoTec will be the dynamic commercialization of the innovative Biomarkers. This will include the market entry and expansion in the US and other key countries. I want to thank Dr. Kilger for her enthusiastic service as Chair of the Advisory Board over many years. Her profound insight in protecting our scientific discoveries laid the ground for what is now possible – building a global business. I am looking forward to working with Dr. Bergmann and Dr. Moesslang in making this happen.” Previously, Dr. Möller spent 23 years at Boehringer Mannheim Group, where he held several senior management positions, including CEO of the worldwide operating Group. Following the company’s acquisition by Roche, Dr. Möller was named Head of Global Development and Strategic Marketing Pharmaceuticals, and a member of Roche’s Executive Committee. He has been an investment advisor at HBM Partners for 12 years and an active board member of many innovative life science companies such as Illumina Inc, B.R.A.H.M.S. AG, MTM Laboratories AG and Morphosys AG. From 2003 to 2016, Dr. Moeller served as Chairman of FIND (Foundation for Innovative New Diagnostics), a product development and implementation partnership financed by the Bill and Melinda Gates Foundation. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform. SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) produces the tests alongside a broad menu of established and commonly used tests for acute and critical care. Contact Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press[at]sphingotec.com www.sphingotec.com

Dr. Angelo Moesslang was named CFO and Managing Director of SphingoTec. The company has developed innovative biomarkers for critical care settings and makes them available on their point of care platform, Nexus IB10. The new senior leadership addition broadens SphingoTec’s core capabilities in response to the anticipated significant expansion of its activities. Hennigsdorf/Berlin, Germany, June 10, 2021 – Diagnostics company SphingoTec GmbH (“SphingoTec”) announces the appointment of Dr. Angelo Moesslang as new chief financial officer (CFO) and managing director. SphingoTec develops and markets innovative in vitro diagnostic solutions for novel biomarkers that support personalized medicine in critical care conditions. SphingoTec is a fully integrated diagnostics company: the innovative biomarkers are commercially available on the proprietary point of care platform Nexus IB10. Following a successful implementation of the biomarkers in clinical routine, the company is now oriented towards geographical expansion and commercial growth. Dr. Angelo Moesslang commented, “I was drawn to SphingoTec’s vision and focus of translating the science of novel biomarkers into clinical routine. I am excited to join such an innovative young company, ideally positioned for growth. This new role will allow me to leverage my expertise, contribute to the commercialization effort and continue the initiatives of improving patient management in critical care with innovative diagnostic solutions.” Dr. Moesslang has more than 20 years of global healthcare and finance leadership experience. Prior to joining SphingoTec he served as CEO of a US-based medical device startup and held various executive roles with the Fresenius Group in Europe, Asia, and the US, most recently CFO of Fresenius Medical Care North America. Dr. Andreas Bergmann, CEO and founder of SphingoTec added, “With the international commercialization of our validated biomarkers and established point of care platform, we are now bringing diagnostic breakthroughs to critical care clinicians. We are excited to welcome Mr. Moesslang, whose entrepreneurial spirit and energy will bring strategic value to our next stage of business development. “ About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform. SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) produces the tests alongside a broad menu of established and commonly used tests for acute and critical care. Contact Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press[at]sphingotec.com www.sphingotec.com

SphingoTec to expand the commercialization strategy by establishing a sales division and appointing new head of sales Tailored diagnostic solutions for point of care use Innovative proprietary biomarkers and standard parameters available on the fully automated Nexus IB10 platform Hennigsdorf/Berlin, Germany, May 20, 2021 – Diagnostics company SphingoTec GmbH (“SphingoTec”) announces an expansion of its go-to-market strategy to scale its sales in Germany by creating a direct sales force for its integrated diagnostic solutions. SphingoTec will sell directly to healthcare providers its Nexus IB10 point of care platform together with a broad portfolio of assays. The rapid tests include commonly used parameters as well as innovative and proprietary biomarkers for critical care settings. The direct sales team will be complementing the already established distributor network and will drive commercialization going forward. The Nexus IB10 platform provides an end-to-end solution for critical care settings that can perform up to three diagnostic parameters on one test disc in only 20 minutes using whole blood samples. The Nexus IB10 analyzer addresses a growing demand for rapid point of care testing in hospital departments, such as intensive care units, emergency rooms, and cath labs. Nexus IB10 is complemented by SphingoTec’s novel biomarker portfolio and targets critical conditions such as sepsis, myocardial infarction, or heart failure. The innovative biomarkers include the kidney function biomarker Proenkephalin (penKid), the endothelial function biomarker bioactive Adrenomedullin (bio-ADM), and the cardiac depression biomarker Dipeptidyl Peptidase 3 (DPP3). To drive growth in the German healthcare market, SphingoTec has appointed Steffen Hohmann to lead the direct sales efforts in Germany. Mr. Hohmann brings 30 years of successful leadership experience working with clinicians, important purchasing groups, and hospital chains. “I am excited to be part of the journey of bringing diagnostic innovation to clinical routine. I am looking forward to increasing the installed base of point of care solutions for improved patient care,” said Hohmann. Dr. Andreas Bergmann, CEO and founder of SphingoTec, said, “Following a successful translation of our novel biomarkers from validation to clinical practice, we are now focusing on actively commercializing our diagnostic solutions. Direct access to our end-users is a key strategic initiative. It enables SphingoTec to serve the critical care community's needs better and help improve care for critically ill patients. We are delighted to have Steffen Hohmann on board to lead our sales initiatives in 2021 and beyond.” About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform. SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) produces the tests alongside a broad menu of established and commonly used tests for acute and critical care. About Nexus Dx Inc. and the Nexus IB10 Platform Nexus Dx Inc., a wholly-owned subsidiary of SphingoTec, headquartered in San Diego, CA, USA, is a global provider of a near patient testing system and advanced diagnostic solution. The company is improving patient care by providing the medical community with rapid and reliable information at the point of care (POC), delivering patient information when and where it is needed most. The company has invested over $160m to develop and market the IB10 analyser system which, without the need for sample preparation, automatically separates plasma from whole blood with subsequent reliable and quantitative detection of biomarkers in the plasma by means of antibodies. With a hands-on-time of less than 3 minutes the easy-to-use system provides in only 20 minutes test results for biomarkers that are crucial in the management of critical care patients. The portfolio of IB10 assays includes tests for established critical care parameters such as Procalcitonin, Troponin I, CK-MB, Myoglobin, NT-proBNP, and D-Dimer as well as tests for SphingoTec’s proprietary biomarkers such as DPP3, a unique and proprietary biomarker for cardiac depression, Proenkephalin (penKid), a unique and proprietary biomarker for real-time assessment of kidney function, and bioactive Adrenomedullin (bio-ADM), a unique and proprietary biomarker for endothelial function. Contact Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press[at]sphingotec.com www.sphingotec.com
Bioactive Adrenomedullin (bio-ADM) is an established biomarker for the real-time assessment of endothelial function in critical care settings Endothelial dysfunction plays a central role in the pathophysiology of COVID-19 Data from the German University Hospital RWTH Aachen show that endothelial function biomarker bio-ADM can support the management of COVID-19 patients Elevated bio-ADM levels indicate disease severity and predict the need for extracorporeal organ assist in COVID-19 patients SphingoTec has made available to the critical care community a rapid point of care test for measuring bio-ADM levels at the point of need Aachen, Germany and Hennigsdorf/Berlin, Germany, May 4, 2021 – German University Hospital Uniklinik RWTH Aachen (“Uniklinik RWTH Aachen”) and diagnostics company SphingoTec GmbH (“SphingoTec”) today announced that the endothelial function biomarker bio-ADM aids in the early risk stratification and management of patients suffering from severe COVID-19, in need for escalated intensive care treatment (1). A team lead by the clinical researchers at Uniklinik RWTH Aachen has shown that high bio-ADM levels indicate the severity of the acute respiratory distress syndrome (ARDS), the subsequent need for organ support, and poor outcomes in severely ill COVID-19 patients. Bioactive Adrenomedullin (bio-ADM) is a hormone regulating endothelial function and has been previously validated in over 35.000 patients with diseases where endothelial dysfunction plays a key role, such as sepsis, septic shock, cardiogenic shock, and acute heart failure. According to the recently published data (1), high bio-ADM levels predict a severe course of COVID-19 infection at admission and identify patients with deteriorating lung function at high risk to develop severe ARDS. Furthermore, the data demonstrate that high bio-ADM levels identify those patients in need of extracorporeal organ assist, such as mechanical ventilation, veno-venous extracorporeal membrane oxygenation (ECMO), renal replacement therapy (RRT). Prof. Gernot Marx, (Director of the Department of Operative Intensive Care Medicine and Intermediate Care at Uniklinik RWTH Aachen) commented, “In the management of COVID-19 patients there is a high need for early risk stratification biomarkers that allows us to allocate resources and timely plan for therapy escalation. Endothelial dysfunction is a central mechanism in the pathophysiology of many critical care conditions, including COVID-19. The information provided by the bio-ADM measurements has convinced us to include it in our clinical routine measurements. “ These findings confirm and extend previous studies in critical care patients where bio-ADM indicates severe complications in sepsis (2,3), acute heart failure (4), and cardiogenic shock (5). High bio-ADM levels indicate severe hypotension, edema formation, ionotropic/vasopressor use, need for organ support, such as mechanical ventilation, and subsequent organ failure. Being a dynamic biomarker, low or decreasing bio-ADM blood levels indicate improved outcomes (3). Dr. Andreas Bergmann, CEO and founder of SphingoTec, said, “By monitoring the endothelial dysfunction early in COVID-19 patients, clinicians can timely identify those patients with an unfavorable disease progression. To further support the management of critically ill patients, we have made available the assay for bioactive Adrenomedullin as a rapid near-patient test.” The CE marked point of care assay for measuring bio-ADM levels is commercialized under the brand name IB10 sphingotest® bio-ADM®. The rapid test runs on SphingoTec`s automated Nexus IB10 point-of-care platform and quantitatively measures levels of bio-ADM directly in blood samples and delivers results after 20 minutes. References (1) Simon T-P et al. Prognostic Value of Bioactive Adrenomedullin in Critically Ill Patients with COVID-19 in Germany: An Observational Cohort Study. Journal of Clinical Medicine. 2021, 10, 1667. doi.org/10.3390/jcm10081667 (2) Mebazaa A et al. Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) study. Critical care. Dec 21 2018;22(1):354. (3) Caironi P et al. Circulating Biologically Active Adrenomedullin (bio-ADM) Predicts Hemodynamic Support Requirement and Mortality During Sepsis. Chest. Aug 2017;152(2):312-320. (4) Ter Maaten JM et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. European journal of heart failure. Jun 2019;21(6):732-743. (5) Tolppanen H et al. Adrenomedullin: a marker of impaired hemodynamics, organ dysfunction, and poor prognosis in cardiogenic shock. Annals of intensive care. Dec 2017;7(1):6. Uniklinik RWTH Aachen The Uniklinik RWTH Aachen is a supramaximal care provider that combines patient-oriented medicine and nursing, teaching and research at an international level. With 36 specialist clinics, 28 institutes and five interdisciplinary units, the University Hospital covers the entire medical spectrum. Excellently qualified teams of doctors, nurses and scientists are competently committed to the health of the patients. The bundling of patient care, research and teaching in one central building offers the best conditions for intensive interdisciplinary exchange and close clinical and scientific networking. Around 8.000 employees provide patient-oriented medicine and care according to recognized quality standards. With 1.400 beds, the University Hospital treats around 50.000 inpatient and 200.000 outpatient cases per year. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform.SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) produces the tests alongside a broad menu of established and commonly used tests for acute and critical care. About bio-ADM IB10 sphingotest® bio-ADM® is a rapid point-of-care (POC) immunoassay for the in vitro quantitative determination of bioactive Adrenomedullin (bio-ADM), a hormone maintaining endothelial function. The endothelium contributes to blood pressure and separates blood from the surrounding tissue. Elevated blood levels of bio-ADM predict blood pressure break down and leaky vessels resulting in oedema. Imbalanced endothelial function is the major cause of shock ultimately resulting in organ dysfunction and death. Early identification of an imbalance in endothelial function allows guidance of vasopressor and diuretic therapy in critically ill patients to improve outcomes. Press contact Dr. Mathias Brandstädter Leitung Unternehmenskommunikation Uniklinik RWTH Aachen Pauwelsstraße 30 52074 Aachen Telefon: 0241 80-89893 mbrandstaedter@ukaachen.de www.ukaachen.de Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.com www.sphingotec.com
DPP3 has been shown to be a cardiac depression factor and a marker for refractory shock DPP3 outperforms Lactate and Procalcitonin on the short-term prognosis in sepsis Changes in the DPP3 levels indicate the worsening or improving of the patient’s condition DPP3 markedly guides intensivists in the management of septic patients Hennigsdorf/Berlin, Germany, March 23, 2021 – Diagnostics company SphingoTec GmbH (“SphingoTec”) announced today the first published data (1) on the biomarker DPP3 that can predict the evolution of organ function and survival in septic patients. Measured on top of routinely used standard parameters, such as Lactate and Procalcitonin, DPP3 is an early indicator of short-term outcomes and patient severity. Sepsis is a medical emergency caused by a dysregulated host response to an infection, with mortality rates increasing rapidly for each hour that appropriate treatment is delayed (2). The rapid evolution of sepsis into its severe form, septic shock, raises the need for more precise and faster testing to support better clinical decision-making. DPP3 is an enzyme at the core of a newly discovered disease mechanism responsible for cardiovascular depression and short-term organ failure in critically ill patients. Although intracellular DPP3 is involved in normal metabolic processes (3), massive cell death leads to DPP3 release into the bloodstream. Circulating DPP3 inactivates Angiotensin II, a hormone regulating the renin-angiotensin-aldosterone system (RAAS), which ultimately controls hemodynamics (4,5). Angiotensin II depletion leads to cardiovascular depression (6,7,9,10) and reduced vascular tone (6,8), a hemodynamic instability that quickly escalates in multiple organ failure. DPP3 was already shown to add value in various critical care settings such as cardiogenic shock (7,9) and burn shock (10). The data from the prospective observational multi-center AdrenoOSS-1 study enrolling about 600 patients with sepsis and septic shock have shown that DPP3 levels can predict multiple short-term organ failure and the need for organ support therapies in this population (1). High or increasing DPP3 blood levels precede organ injury and predict the need for vasopressor/inotropic use, mechanical ventilation, and renal replacement therapy. DPP3 blood levels also reflect patient severity, with septic shock patients having a significantly higher DPP3 concentration than patients with severe sepsis (1,3). The study data further show that low or decreasing levels of DPP3 in the first 24 hours of ICU admission predict an improvement of organ function and better outcomes. The dynamic nature of the biomarker can guide intensivists in the early management of septic shock patients. Dr. Andreas Bergmann, founder of various companies, where new tools to fight sepsis mortality are developed, and CEO of critical care diagnostics company SphingoTec commented: “This newly discovered biomarker makes it easy to identify a distinct pathophysiological mechanism leading to mortality in sepsis and uncovers the etiology of clinical symptoms. The published data show that measuring DPP3 levels add value in the clinical practice by early revealing organ injury and adding information on top of standard parameters. This can help guide intensivists in the early management of septic patients.“ The in vitro diagnostic test for DPP3 is available as a microtiter plate as well as a near-patient rapid test (IB10 sphingotest® DPP3). SphingoTec has made the test available for the critical care community to further assess the clinical utility of the DPP3 biomarker in acute care settings. References 1. Blet et al (2021), Monitoring Circulating dipeptidyl peptidase 3 (DPP3) predicts improvement of organ failure and survival in sepsis: a prospective observational multinational study, Crit Care, DOI: https://doi.org/10.1186/s13054-021-03471-2 2. Bai et al (2014) Early versus delayed administration of norepinephrine in patients with septic shock Crit Care..DOI:https://doi.org/10.1186/s13054-014-0532-y 3. Rehfeld et al (2019). Novel Methods for the Quantification of Dipeptidyl Peptidase 3 (DPP3) Concentration and Activity in Human Blood Samples. J Appl Lab Med. DOI: 10.1373/jalm.2018.027995 4. Picod et al (2020), Alteration of the Renin-Angiotensin-Aldosterone System in Shock: Role of the Dipeptidyl Peptidase 3, Am J Respir Crit Care Med, 2020, DOI: 10.1164/rccm.202010-3873LE 5. Jha et al (2020), Dipeptidyl peptidase 3 modulates the renin–angiotensin system in mice, J Biol Chem., DOI: 10.1074/jbc.RA120.014183. 6. Deniau et al.(2020) Inhibition of circulating dipeptidyl-peptidase 3 restores cardiac function in a sepsis-induced model in rats: A proof of concept study. PloS One. DOI: doi.org/10.1371/journal.pone.0238039 7. Takagi et al.(2019) Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: results from the OptimaCC trial. Eur J Heart Fail., DOI: 10.1002/ejhf.1600 8. van Lier (2020), Promotion of vascular integrity in sepsis through modulation of bioactive adrenomedullin and dipeptidyl peptidase 3, J. Intern. Med., DOI: doi.org/10.1111/joim.13220 9. Deniau et al. (2019), Circulating dipeptidyl peptidase is a myocardial depressant factor: dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur J Heart Fail. DOI : 10.1002/ejhf.1601 10. Dépret et al.(2020) Circulating dipeptidyl peptidase-3 at admission is associated with circulatory failure, acute kidney injury and death in severely ill burn patients. Crit Care , DOI: https://doi.org/10.1186/s13054-020-02888-5 ## About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. www.sphingotec.com About DPP3 sphingotest® DPP3 measures Dipeptidyl peptidase 3 an active enzyme which, when released into the blood, inactivates Angiotensin II, a hormone that is important for heart function. The depletion of Angiotensin II affects the renin-angiotensin-aldosterone system (RAAS), ultimately leading to cardiovascular depression and reduced vascular tone, a deadly combination in need of selective treatment strategies. The DPP3 release is a newly identified disease mechanism explaining short-term organ failure in critically ill patients. Early identification of DPP3 release may allow better patient stratification and earlier therapy escalation to improve outcomes. Press contact Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press@sphingotec.com www.sphingotec.com
Researchers have published new insights into the causes of mortality in sepsis Loss of endothelial function is induced through two different pathophysiological processes and is a major driver of septic shock, a life-threatening drop in blood pressure The first pathway originates in the loss of the endothelial barrier triggering an increased production of the repair hormone bioactive Adrenomedullin (bio-ADM), which also has the undesired side effect of vasodilation The second threat acting on the endothelial function is the release of the protease DPP3 into the bloodstream which degrades angiotensin II, a process resulting in decreased vascular tone and cardiac output The different pathways require different treatment strategies thus opening new approaches for personalized medicine in sepsis New diagnostics for quantification of bio-ADM and DPP3 are available as laboratory and near-patient rapid tests Biomarker-guided approaches for therapies targeting these pathways are showing promising results Hennigsdorf/Berlin, Germany, February 18, 2021 – Diagnostics company SphingoTec GmbH (SphingoTec) announced today that two distinct processes are involved in the development of septic shock and that SphingoTec’s biomarkers for endothelial function (vascular integrity) and cardiovascular depression allow early identification of these underlying mechanisms requiring different interventions. Sepsis, a global burden with nearly 50 million cases worldwide, is a life-threatening condition that is diagnostically and therapeutically underserved. With the availability of such pathway-specific biomarkers, new avenues for diagnosing and monitoring sepsis are opened and biomarker-guided trials for personalized therapies targeting these mechanisms are enabled. Researchers have summarized the available evidence (1) on two distinct pathophysiological processes leading to endothelial dysfunction and the subsequent development of shock and organ failure in sepsis. The two biologically active molecules acting on the vasculature and influencing patient outcomes are bioactive Adrenomedullin (bio-ADM) and Dipeptidyl peptidase 3 (DPP3). One distinct pathway originates in the loss of endothelial barrier integrity, causing edema and the loss of intravascular volume. To compensate for this leakage, the production of the repair hormone bio-ADM is increased. But bio-ADM has also the second function of vascular relaxation, therefore the increased production leads to a dangerous side effect of vasodilation, generating a loss of tissue resistance which ultimately culminates in shock. Data from the observational study AdrenOSS-1 show (3) that elevations of bio-ADM levels reflect the loss of endothelial function and translate into poor outcome in sepsis. Furthermore, the results of the biomarker-guided AdrenOSS-2 trial (2) confirm that this pathway is a valid therapeutic target. According to the second underlying mechanism accountable for the loss of the endothelial function, the depletion of angiotensin II affects the renin-angiotensin-aldosterone system (RAAS), ultimately leading to a cardiovascular depression (4,5) and reduced vascular tone, a deadly combination in need of selective treatment strategies. The main process generating the depletion of the cardiovascular stimulating hormone angiotensin II is the release of the protease DPP3 into the bloodstream through sepsis-induced cell damage (6). Personalized medicine has shown significant progress in areas such as oncology or cardiology, but in intensive care units, it has remained challenging to identify biomarkers that facilitate personalized treatments. In the context of a life-threatening condition such as septic shock, taking therapeutic decisions is time-critical, aiming to respond in the best possible way and especially on a patient-specific basis. The review (1) summarizes that the biomarkers bio-ADM and DPP3 can identify these pathways, supporting an early and precise diagnosis and monitoring of sepsis patients. Moreover, data from the biomarker-guided interventional study AdrenOSS-2 show that clinical trials can benefit from the use of biomarker as an enrichment strategy. Within the AdrenOSS-2 study, patients with sepsis-associated endothelial dysfunction were identified by increased bio-ADM to receive therapy with placebo or Adrecizumab (2), an antibody targeting the loss of vascular integrity by maintaining protective bio-ADM concentrations in the blood. When excluding patients with additionally high DPP3 blood concentrations, outcomes could further be improved. Therapies blocking DPP3-activity have also been shown to improve outcomes in various preclinical models. (7) Dr. Andreas Bergmann, founder of various companies fighting sepsis mortality and CEO of critical care diagnostics company SphingoTec commented: “Following a deep understanding of the disease biology, we have developed diagnostic solutions that can now unravel the etiology of the mortality drivers in sepsis. The evidence confirms the utility of our biomarkers in supporting clinicians make more informed decisions and ultimately improve patient management. “ The new diagnostics for quantification of bio-ADM and DPP3 are available as microtiter plate assays as well as point-of-care tests on the Nexus IB10 immunoassay platform. The Nexus IB10 analyzer provides test results on whole blood samples in only 20 minutes and can be flexibly deployed in emergency departments, intensive care units, and any laboratory setting. References (1) van Lier (2020), Promotion of vascular integrity in sepsis through modulation of bioactive adrenomedullin and dipeptidyl peptidase 3, J. Intern. Med., DOI: doi.org/10.1111/joim.13220 (2) Geven et al (2019)A double-blind, placebo-controlled, randomised, multicentre, proof-of-concept and dose-finding phase II clinical trial to investigate the safety, tolerability and efficacy of adrecizumab in patients with septic shock and elevated adrenomedullin concentration (AdrenOSS-2), BMJ, DOI: 10.1136/bmjopen-2018-024475 (3) Mebazaa (2018), Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) study , Crit Care DOI: (4) Jha et al (2020), Dipeptidyl peptidase 3 modulates the renin–angiotensin system in mice, J Biol Chem ., DOI: 10.1074/jbc.RA120.014183. (5) Takagi et al (2020), Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: results from the OptimaCC trial, Eur J Heart Fail, DOI: 10.1002/ejhf.1600 (6) Bet et al (2021) Monitoring circulating dipeptidyl peptidase 3 (DPP3) predicts improvement of organ failure and survival in sepsis: a prospective observational multinational study. Crit Care DOI: doi.org/10.1186/s13054-021-03471-2 (7) Deniau et al (2020) Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics, Eur J Heart Fail, DOI: 10.1002/ejhf.1601. ### About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM), a biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid), a biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. About bio-ADM® sphingotest® bio-ADM® measures bioactive Adrenomedullin (bio-ADM), a hormone maintaining endothelial function. The endothelium contributes to blood pressure and separates blood from the surrounding tissue. Elevated blood levels of bio-ADM® predict blood pressure break down and leaky vessels resulting in oedema. Imbalanced endothelial function is the major cause of shock ultimately resulting in organ dysfunction and death. Early identification of an imbalance in endothelial function allows guidance of vasopressor and diuretic therapy in critically ill patients to improve outcomes. Learn more about bio-ADM® at www.sphingotec.com About DPP3 sphingotest® DPP3 measures Dipeptidyl peptidase 3 an active enzyme which, when released into the blood, inactivates angiotensin II, a hormone that is important for heart function. The depletion of angiotensin II affects the renin-angiotensin-aldosterone system (RAAS), ultimately leading to cardiovascular depression and reduced vascular tone, a deadly combination in need of selective treatment strategies. The DPP3 release is a newly identified disease mechanism explaining short-term organ failure in critically ill patients. Early identification of DPP3 release may allow better patient stratification and earlier therapy escalation to improve outcomes. www.sphingotec.com

The latest consensus meeting of international experts in critical care and nephrology supports the use of novel biomarkers in the prevention and management of Acute Kidney Injury (AKI) The consensus recommends using a combination of damage and functional biomarkers together with clinical information for routine practice Proenkephalin (penKid®), the kidney function biomarker, proposed as a marker for the assessment of AKI progression and kidney recovery The CE-IVD marked assay for penKid® is available for point of care usage on the fully automated Nexus IB10 platform Hennigsdorf/Berlin, Germany, December 16, 2020 – Diagnostics company SphingoTec GmbH (“SphingoTec”) announced today that the Acute Disease Quality Initiative (ADQI) recommends the use of novel biomarkers for AKI, including functional biomarkers as penKid®. Since AKI is affecting 1 in 3 Intensive Care Unit (ICU) patients [1], and the current standard of care diagnostics has considerable sensitivity and specificity limitations, there is an urgent need to implement new biomarkers to assist a better management of AKI. The current consensus recommendations [2] support clinicians in making more informed decisions and improve outcomes with biomarker guided management of AKI patients, including triage, diagnosis, and guidance of therapy. Among the main recommendation of the ADQI meeting is the use of novel biomarkers to assess AKI progression and kidney recovery. The consensus statements highlight the performance and added value of penKid® for the prediction of duration and recovery of AKI. Based on the results of a multicenter trial (3), the presented evidence shows that penKid® concentration is significantly lower in AKI patients with improving kidney function when compared to patients without kidney recovery. Additional data (4) is used by the experts in ADQI to convey that significantly higher penKid® levels are indicating those patients with major adverse kidney events, patients with persistent AKI, and those who had worsening of kidney function. Furthermore, the consensus statement also underlines that penKid® is an earlier biomarker than today’s standard of care diagnostics in identifying the patients with worsening kidney function. Prof. Peter Pickkers (Radboud University, Nijmegen), member of the ADQI explained “Since the last evaluation of novel AKI biomarkers 9 years ago, we have collected enough evidence now to consider the usage of functional and damage biomarkers in the prediction and management of AKI. Although many novel biomarkers can measure the damage that already occurred in the kidneys, there are few choices available for measuring the kidney function. Besides Cystatin C, penKid is the only novel functional biomarker available for clinical routine practice.” The kidney function biomarker penKid® was previously validated in over 40.000 patients and published data [5] demonstrate that penKid® can detect the presence and severity of AKI and enables the identification of patients at high risk of unfavorable outcomes. Moreover, previous findings from the AdrenOSS 1, a 24-centers study, show that penKid® not only diagnoses AKI earlier than today’s standard of care, but it also indicates the renal recovery. [4] The utility of penKid® has been proved both in adult and children population. [6] Dr. Andreas Bergmann, CEO and founder of SphingoTec stated “We are excited that penKid® has been recognized by ADQI as a suitable functional kidney biomarker. Not only that penKid® reflects kidney function and true GFR independent of inflammation and comorbidities, but unpublished clinical data could complement the consensus recommendation to further evaluate the functional biomarker’s role in defining the optimal timing for initiating and stopping kidney replacement therapy.” To support timely treatment decisions that are likely to improve patient management in critical care patients, SphingoTec makes available the CE-IVD marked assay for penKid® on its proprietary Nexus IB10 platform. The fully automated point-of-care analyzer uses whole blood, delivers results in only 20 minutes, and can be flexibly deployed in near-patient as well as laboratory settings. References: (1) Ponce et al (2016), Acute kidney injury: risk factors and management challenges in developing countries, Int J Nephrol Renovasc Dis., DOI: 10.2147/IJNRD.S104209 (2) Ostermann et al (2020), Recommendation on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference | Consensus Statement, Critical Care Medicine, DOI: 10.1001/jamanetworkopen.2020.19209 (3) Caironi et al (2018), Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem; DOI:10.1373/clinchem.2018.288068 (4) Hollinger et at (2018), Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study, Kidney Int Rep, DOI: 10.1016/j.ekir.2018.08.006 (5) Marino et al (2015), Diagnostic and short-term prognostic utility of plasma proenkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department, J Nephrol, DOI 10.1007/s40620-014-0163-z [6] Hartman et al (2020), Proenkephalin as a New Biomarker for Pediatric Acute Kidney Injury - Reference Values and Performance in Children Under One Year of Age, Clin Chem Lab Med, doi: 10.1515/cclm-2020-0381. About penKid® IB10 sphingotest® penKid® measures Proenkephalin (penKid®), a stable fragment of the kidney stimulating hormone Enkephalin. penKid® has been demonstrated to be a real-time surrogate biomarker for glomerular filtration rate, the gold standard to assess renal function. Measuring penKid® blood concentrations allows for timely information on kidney function in critically ill patients. Early assessment of worsening and improving of renal function on intensive care units and in emergency departments allows adjustment of nephrotoxic drug administration and the initiation of kidney-protective strategies to prevent acute kidney injury and thereby improve outcomes. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. In addition, SphingoTec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. About Nexus Dx Inc. and the IB10 Platform Nexus Dx Inc., a wholly-owned subsidiary of SphingoTec, headquartered in San Diego, CA, USA, is a global provider of a near patient testing system and advanced diagnostic solution. The company is improving patient care by providing the medical community with rapid and reliable information at the point of care (POC), delivering patient information when and where it is needed most. The company has invested over $160m to develop and market the IB10 analyzer system which, without the need for sample preparation, automatically separates plasma from whole blood with subsequent reliable and quantitative detection of biomarkers in the plasma by means of antibodies. With a hands-on-time of less than 3 minutes the easy-to-use system provides in only 20 minutes test results for biomarkers that are crucial in the management of critical care patients. The portfolio of IB10 assays includes tests for established critical care parameters such as Procalcitonin, Troponin I, CK-MB, Myoglobin, NT-proBNP, and D-Dimer as well as tests for SphingoTec’s proprietary biomarkers such as Proenkephalin (penKid®), a unique and proprietary biomarker for real-time assessment of kidney function, and bioactive Adrenomedullin (bio-ADM®), a unique and proprietary biomarker for endothelial function. Contact SphingoTec GmbH Ruxandra Lenz Sr. Manager Marketing and Communications Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com

The biomarkers for the real-time assessment of kidney function (penKid®) and endothelial function (bio-ADM®) are pioneering new diagnostic pathways in critical care settings Following a positive initial clinical evaluation of penKid® and bio-ADM®, the University Hospital RWTH Aachen has transferred the innovative diagnostic tools into clinical routine The novel biomarkers enable a better diagnosis and monitoring of organ function Aachen, Germany and Hennigsdorf/Berlin, German, December 10, 2020 – The Uniklinik RWTH Aachen (“Uniklinik RWTH Aachen”) has successfully translated the collaboration for research and biomarker validation with SphingoTec GmbH (“SphingoTec”) into clinical routine. The routine measurements of the innovative biomarkers are providing organ-specific information for monitoring critical care conditions such as sepsis and acute kidney injury and support clinical decisions to improve patient outcomes. Uniklinik RWTH Aachen is one of Germany’s most modern hospitals due to the way it integrates diagnostics and therapy, research and teaching under the same roof. Following a patient-centric approach, Uniklinik RWTH Aachen has adopted innovative pathways in intensive medicine by introducing these new diagnostic tools for monitoring organ function of critically ill patients. The routine measurements of penKid® and bio-ADM® provide clinicians with more insights on the disease pathology and etiology of clinical symptoms and facilitate more efficient, timely, and adequate treatment. Prof. Dr. Gernot Marx, the Director of the Clinic for Operative Intensive Care and Intermediate Care at Uniklinik RWTH Aachen explained: “Critically ill patients are highly dynamic with many complications interfering in the diagnostics process, thus a very challenging environment for introducing innovations. We have been looking for a long time for the right diagnostic tools to allow us a faster and better diagnosis, risk stratification, and monitoring of the disease progression so that we can provide the best available treatment immediately for acute cases. The first routine measurements do confirm the utility and value of these novel diagnostic biomarkers in clinical decision-making and ultimately in maximizing the patient’s benefit. “ In intensive care units, 1 in 3 patients is developing acute kidney injury [1]. The existing diagnostic parameters for the determination of renal function or kidney damage, which are routinely used as standard procedure, have a considerable time delay or are influenced by inflammation or other diseases. These limitations are underlining the need for more precise tools to support clinical decisions. The biomarker penKid® offers real-time information about the kidney function with the first measurement and without being influenced by co-morbidities or the frequently occurring inflammation in critically ill patients [2,3,4]. Moreover, penKid® shows the best representation of the current kidney function, measured by the glomerular filtration rate (true GFR). [5] World-wide, sepsis is accountable for 1 in 5 deaths [6]. Reduced organ perfusion in shock, which can be determined by existing laboratory values, is the culprit for the fatal course, but it can be induced by various factors. The loss of endothelial function is often a main cause for shock in sepsis, but it could not be detected by blood-based tests so far. The measurement of the bio-ADM® biomarker now allows for the first time the direct assessment of endothelial function in real time [7,8,9] independent of co-morbidities and inflammation, thus supporting precise and rapid treatment decisions. Dr. Andreas Bergmann, founder and CEO of SphingoTec added: “We are excited that the initial clinical evaluation ended in a successful translation of the biomarkers into clinical routine. The comprehensive amount of routine data on our biomarkers collected by Prof. Gernot Marx and his team will allow us to deepen our knowledge of the biomarkers and to explore further application areas. Encouraged by this implementation of our novel diagnostic tools at the frontlines of medical care, we intend to offer them in a near future on a larger scale in other European countries.” References [1] Ponce et. al. (2016), Acute kidney injury: risk factors and management challenges in developing countries Int. J. Nephrol. Renovasc. Dis., DOI: 10.2147/IJNRD.S104209 [2] Hollinger et. al. (2018) Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study, Kidney Int Rep, DOI: 10.1016/j.ekir.2018.08.006 [3] Siong Chan (2018) Proenkephalin in heart failure [4] Beunders et. al. (2017) Proenkephalin (PENK) as a novel biomarker for kidney function. [5] Beunders, R. et al. (2019), Proenkephalin compared to conventional methods to assess kidney function in critically ill sepsis patients, Shock,, doi:10.1097/SHK.0000000000001510 (6) Rudd et al (2020), Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study, The Lancet, DOI: doi.org/10.1016/S0140-6736(19)32989-7 (7) Geven (2018): Vascular Effects of Adrenomedullin and the anti-Adrenomedullin Antibody Adrecizumab in Sepsis. Shock. doi: 1097/SHK.0000000000001103 (8) Mebazaa (2018): Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock (AdrenOSS-1) study. doi:10.1186/s13054-018-2243-2 (9) Caironi (2017): Circulating biologically active adrenomedullin (bio-ADM) predicts hemodynamic support requirement and mortality During Sepsis. Chest. doi: 10.1016/j.chest.2017.03.035. Uniklinik RWTH Aachen The Uniklinik RWTH Aachen is a supramaximal care provider that combines patient-oriented medicine and nursing, teaching and research at an international level. With 36 specialist clinics, 28 institutes and five interdisciplinary units, the University Hospital covers the entire medical spectrum. Excellently qualified teams of doctors, nurses and scientists are competently committed to the health of the patients. The bundling of patient care, research and teaching in one central building offers the best conditions for intensive interdisciplinary exchange and close clinical and scientific networking. Around 8.000 employees provide patient-oriented medicine and care according to recognised quality standards. With 1.400 beds, the University Hospital treats around 50.000 inpatient and 200.000 outpatient cases per year. About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes bioactive Adrenomedullin (bio-ADM®), a biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiter plate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. About penKid® sphingotest® penKid® measures Proenkephalin (penKid®), a stable fragment of the kidney stimulating hormone Enkephalin. penKid® has been demonstrated to be a real-time surrogate biomarker for glomerular filtration rate, the gold standard to assess renal function. Measuring penKid® blood concentrations allows for timely information on kidney function in critically ill patients. Early assessment of worsening and improving of renal function on intensive care units and in emergency departments allows adjustment of nephrotoxic drug administration and the initiation of kidney-protective strategies to prevent acute kidney injury and thereby improve outcomes. Learn more about penKid® at http://www.youtube.com/watch?v=6SYhs7it4R4 About bio-ADM® sphingotest® bio-ADM® measures bioactive Adrenomedullin (bio-ADM®), a hormone maintaining endothelial function. The endothelium contributes to blood pressure and separates blood from the surrounding tissue. Elevated blood levels of bio-ADM® predict blood pressure break down and leaky vessels resulting in oedema. Imbalanced endothelial function is the major cause of shock ultimately resulting in organ dysfunction and death. Early identification of an imbalance in endothelial function allows guidance of vasopressor and diuretic therapy in critically ill patients to improve outcomes. Learn more about bio-ADM® at http://www.youtube.com/watch?v=52lrrRNb0k4 Press contact Dr. Mathias Brandstädter Leitung Unternehmenskommunikation Uniklinik RWTH Aachen Pauwelsstraße 30 52074 Aachen Telefon: 0241 80-89893 mbrandstaedter@ukaachen.de www.ukaachen.de Ruxandra Lenz Sr. Manager Marketing and Communications SphingoTec GmbH Neuendorfstr. 15 A 16761 Hennigsdorf Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com

BHR specializes in innovative diagnostic solutions for point of care Collaboration focuses on SphingoTec’s proprietary assays for bio-ADM®, penKid® and DPP3 Investigator-initiated study in COVID-19 at Guy’s & St Thomas’ Hospital and King's College Hospital NHS Trust Warwickshire, United Kingdom and Hennigsdorf, Germany, September 29, 2020 - BHR Pharmaceuticals Ltd (“BHR”) and SphingoTec GmbH (“SphingoTec”) today announced that they have signed a distribution agreement for the commercialization of SphingoTec’s portfolio of diagnostics solutions in acute and critical care in UK and Ireland. The collaboration in particular focuses on the UK market introduction of SphingoTec’s point-of-care tests for novel and proprietary biomarkers in critical care targeting diagnostically underserved conditions such as sepsis, acute heart failure, acute kidney injury, and recently severe COVID-19. These products include tests for bio-ADM®, penKid®, and DPP3 allowing for the early diagnosis and monitoring of endothelial dysfunction, kidney dysfunction, and cardiac depression, respectively. The tests are made available on SphingoTec’s Nexus IB10 platform, a fully automated rapid immunoassay point-of-care instrument, that provides accurate test results within only 20 minutes and can be flexibly deployed in laboratories, emergency departments, intensive care units, and doctors’ offices. Established in 1990, BHR has grown to become a UK market leader in the provision of point-of-care diagnostics. BHR specializes in sourcing new advanced and progressive technology from around the world and delivering these innovative products to the UK market. Bharat Vadukul, Director of BHR commented “BHR is delighted to be appointed exclusive distribution partners for the Nexus IB10 system in the United Kingdom and Ireland. This product further strengthens the strategic fit with our existing product portfolio. With the Nexus IB10 system, BHR’s comprehensive package of Point-of-Care testing devices will enhance the ability of Specialists in the Renal and Cardiology sectors to better manage their patients, saving lives and improve their all-important quality of life.” Dr. Andreas Bergmann, CEO of SphingoTec, echoes those sentiments. He noted, “We are very much looking forward to our collaboration with BHR who have been very successful in promoting the IB10 platform in the past. We also believe that they are excellent strategic partners for providing rapid and novel solutions needed by clinicians in managing critically ill patients and will support us in our long-term strategy to improve patient outcomes in acute and critical care conditions.” To address the specific challenges provided by COVID-19 in the UK, the companies collaborate with Dr. Marlies Ostermann, MD, Ph.D. at Guy’s & St Thomas’ Hospital/London, United Kingdom, who initiated a two-center study also including King's College Hospital NHS Trust to further investigate the added value of SphingoTec’s biomarkers in developing individualized management strategies for severely ill COVID-19 patients(1). Dr. Ostermann commented: “There is an urgent need to assess and monitor organ function of COVID-19 patients in real-time. After a first positive evaluation of these novel biomarkers, we have decided to further investigate their use in clinical practice in delivering information on the underlying pathogenesis in patients with COVID-19. “ To discover more about the Nexus IB10 and SphingoTec’s portfolio of diagnostic solutions for acute and critical care, please visit sphingotec.com. For more information on the BHR Pharmaceuticals portfolio, please visit www.bhr.co.uk (1) clinicaltrials.gov/ct2/show/NCT04408365 About Sphingotec SphingoTec GmbH ( “SphingoTec”; Hennigsdorf near Berlin, Germany) develops and markets innovative in-vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes Bioactive Adrenomedullin(bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. About BHR Pharmaceuticals BHR Pharmaceuticals Ltd is a market leading company in the field of point-of-care diagnostics within healthcare, moving appropriate tests from the lab closer to the patient to better manage and treat people. This helps shorten patient pathways, improves the patient experience, and improves efficiency for doctors, nurses and other healthcare professionals. BHR has challenges the conventions of laboratory medicine by showing that point-of-care diagnostics save time, money and improves health. Contact SphingoTec GmbH Ruxandra Lenz Sr. Manager Marketing and Communications Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com

penKid® (Proenkephalin), a unique biomarker for the real-time assessment of kidney function Novel data now demonstrate that penKid® also accurately predicts acute kidney injury in infants and provides substantial additional value on top of the diagnostic standard of care Hennigsdorf/Berlin, Germany, August 27, 2020 – Diagnostics company SphingoTec GmbH (“SphingoTec”) announced today the publication of first data (1), proving that its real-time kidney function biomarker penKid® is a reliable biomarker for the diagnosis of acute kidney injury (AKI) in infants. With the incidence of pediatric AKI increasing globally, there is a high need for non-invasive, diagnostic solutions to improve the early diagnosis of AKI in children to prevent adverse events and improve recovery.penKid® is an established biomarker for detecting AKI in adults. The current study shows that reference values for penKid® in children are much higher than in adults. However, when considering these higher reference values in critically ill children under one year of age, high or increasing penKid® concentrations accurately predict AKI and its severity. Dr. Andreas Bergmann, CEO of SphingoTec, commented: “The data confirm, that penKid® predicts AKI also in challenging settings such as the pediatric critical care. In critically ill children, AKI has a high prelevance, is associated with poor outcomes and the possibilities for pharmacological intervention are limited. Therefore, the early detection of AKI is paramount in improving the prognosis for these children.” In children admitted to hospital, AKI is a common complication especially among those requiring intensive care, affecting up to 51% admitted to ICU (2). Pediatric AKI is often diagnosed too late for successful therapeutic interventions leading to adverse outcomes and chronic kidney impairment. The data from the recent study highlight that penKid® has the potential to address this diagnostic shortcoming. The study data add to the rapidly growing body of evidence in by now more than 30,000 adult patients, showing that penKid® is an early and accurate indicator for the worsening or improvement of kidney function. Importantly, penKid® is independent of inflammation and comorbidities and, as recently published, the most accurate surrogate marker for true glomerular filtration rate (true GFR) in patients with renal impairment (3) and hence ideally suited for the real-time assessment of kidney function and the early prediction of AKI. References (1) Hartman et al (2020), Proenkephalin as a New Biomarker for Pediatric Acute Kidney Injury - Reference Values and Performance in Children Under One Year of Age, Clin Chem Lab Med, doi: 10.1515/cclm-2020-0381. (2) Sutherland et al (2015) AKI in Hospitalized Children: Comparing the pRIFLE, AKIN, and KDIGO Definitions, Clin J Am Soc Nephrol, doi: 10.2215/CJN.01900214. (3) Beunders, R. et al.(2020), Proenkephalin compared to conventional methods to assess kidney function in critically ill sepsis patients, Shock, doi:10.1097/SHK.0000000000001510 About sphingotec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec’s proprietary biomarker portfolio includes Bioactive Adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. About penKid® sphingotest® penKid® measures proenkephalin (penKid®), a stable fragment of the kidney stimulating hormone enkephalin. penKid® has been demonstrated to be a real-time surrogate biomarker for glomerular filtration rate, the gold standard to assess renal function. Measuring penKid® blood concentrations allows for timely information on kidney function in critically ill patients. Early assessment of worsening and improving of renal function on intensive care units and in emergency departments allows adjustment of nephrotoxic drug administration and the initiation of kidney-protective strategies to prevent acute kidney injury and thereby improve outcomes. Contact SphingoTec GmbH Ruxandra Lenz Sr. Manager Marketing and Communications Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com

Hennigsdorf, Germany August 19, 2020 – Diagnostic company SphingoTec GmbH (SphingoTec) today announced that the Company has issued a non-brokered, unsecured convertible note facility amounting to EUR 5 million that was fully subscribed by current and new investors. The proceeds of the transaction will bridge to a larger financing round planned for later this year or early 2021 to fund the further growth of the company, in particular the commercialization of the company’s innovative diagnostic products for critical care in Europe, the required scale up of manufacturing capabilities and gaining access to the U.S. market. About SphingoTec SphingoTec GmbH ( “SphingoTec”; Hennigsdorf near Berlin, Germany) develops and markets innovative in-vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes Bioactive Adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care.

Hennigsdorf/Berlin, Germany – August 6, 2020 – Diagnostics company SphingoTec GmbH (“SphingoTec”) today announced that Dr Andreas Bergman, CEO, and Ferdinand Von Humboldt, CFO, will be presenting a corporate overview at the Summer Private Company Showcase, hosted by Solebury Trout on August 10, 2020. SphingoTec develops and markets in-vitro diagnostic tests for novel and proprietary biomarkers in diagnostically underserved acute and critical care conditions to improve patient outcomes. The company makes its assays for real-time assessment of kidney function, endothelial function and cardiac depression available on its fully integrated Nexus IB10 point-of-care platform. Presentation Details: Date: August 10, 2020 Time: 4:00 p.m. CEST Please click the following link to register: troutaccess.com/index.php/c/Summer2020PCS About SphingoTec SphingoTec GmbH ( “SphingoTec”; Hennigsdorf near Berlin, Germany) develops and markets innovative in-vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes Bioactive Adrenomedullin(bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care.

Hennigsdorf/Berlin, Germany – July 31, 2020 – Diagnostics company SphingoTec GmbH (“SphingoTec”) today announced that Dr Andreas Bergman, CEO, and Ferdinand von Humboldt, CFO, will be presenting a corporate overview at the Private Healthcare Company Virtual Summer Symposium, hosted by LifeSci Partners on August 4 and 5, 2020. SphingoTec develops and markets in-vitro diagnostic tests for novel and proprietary biomarkers in diagnostically underserved acute and critical care conditions to improve patient outcomes. The company makes its assays for real-time assessment of kidney function, endothelial function and cardiac depression available on its fully integrated Nexus IB10 point-of-care platform. Presentation Details: Date: August 4, 2020 Time: 3:30 p.m. CEST Please click the following link to register: https://lifesci.events/SummerSymposium About SphingoTec SphingoTec GmbH ( “SphingoTec”; Hennigsdorf near Berlin, Germany) develops and markets innovative in-vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes Bioactive Adrenomedullin(bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care.
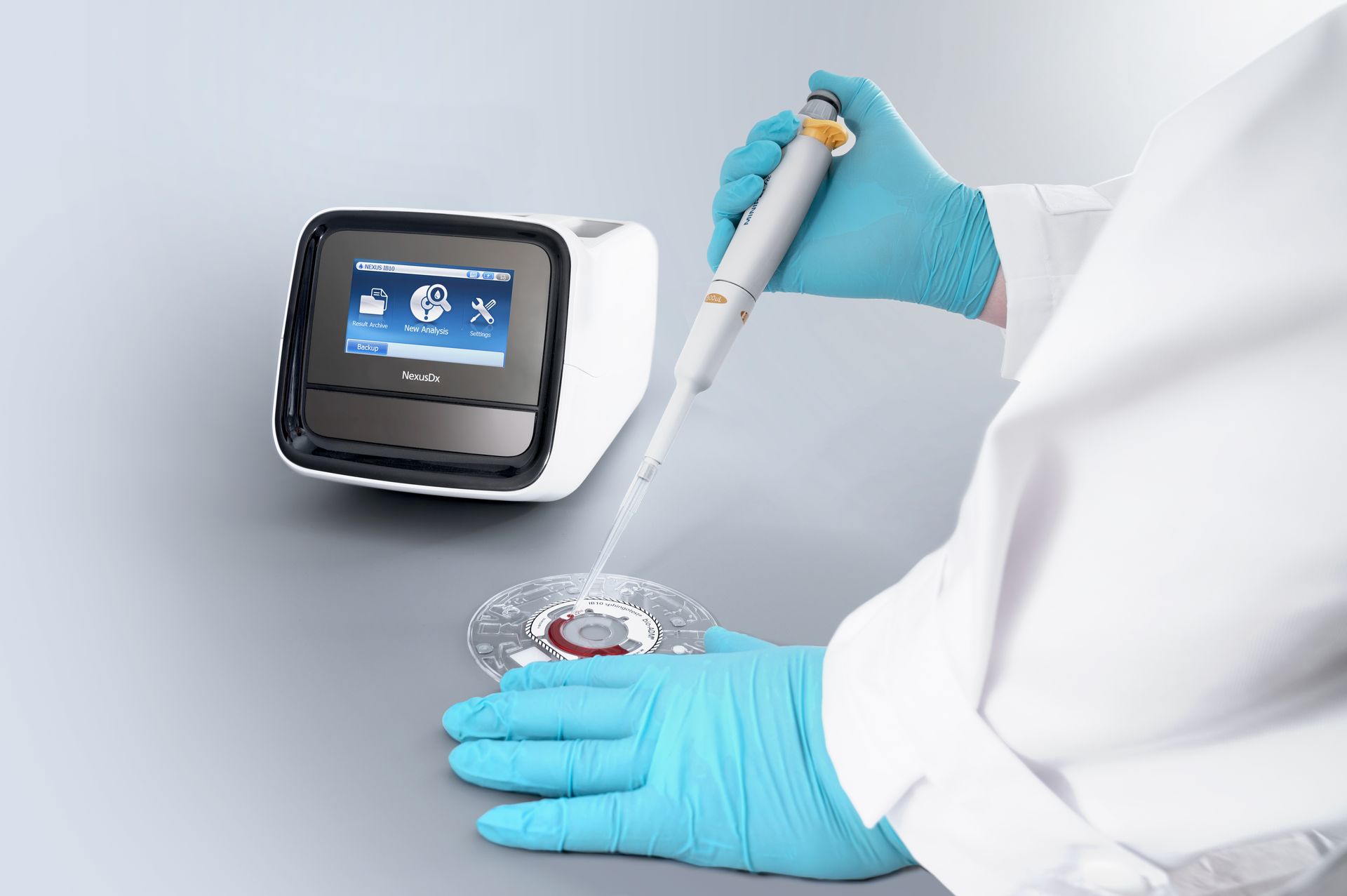
Endothelial dysfunction is driving pathophysiological processes such as congestion in heart failure, shock in sepsis, and lung dysfunction in COVID-19 Bioactive Adrenomedullin (bio-ADM®) is a proprietary biomarker for real-time assessment of endothelial function in acute and critical care settings. High blood levels of bio-ADM® have been shown to predict septic shock, to diagnose residual congestion in heart failure, and the need for organ support in critically ill patients, including COVID-19 IB10 sphingotest® bio-ADM® runs on SphingoTec`s automated Nexus IB10 point-of-care platform and quantitatively measures levels of bio-ADM® directly in blood samples Hennigsdorf/Berlin, Germany, July 22, 2020 – Diagnostics company SphingoTec GmbH (“SphingoTec”) today announced the launch of its IB10 sphingotest® bio-ADM®, a CE-IVD-marked point-of-care test to quantitatively determine blood levels of Bioactive Adrenomedullin (bio-ADM®). Blood levels of bio-ADM® reflect in real-time the functional status of the endothelium, the inner cell sheet of blood vessels. The test is made available on the company`s rapid point-of-care platform Nexus IB10 that uses whole blood samples without any pre-processing, requires less than three minutes hands-on-time, and delivers results after 20 minutes. Nexus IB10 can be flexibly deployed in laboratories or near-patient settings such as intensive care units (ICUs) and Emergency Departments (EDs). In numerous studies on more than 22,000 patients admitted to ICUs and EDs, bio-ADM® has been shown to predict distortions of the inner cell sheet of blood vessels, the endothelium (1). Failure of the endothelial function has been demonstrated to precede edema and the life-threatening blood pressure drop that causes shock and multiorgan failure e.g. in patients with sepsis at ICUs and EDs (2-3). In heart failure patients, bio-ADM® blood-levels reliably and objectively reflect tissue congestion and residual congestion (4). Recent data show that elevated blood levels of this functional biomarker also identifies patients in the general ICU patient population who require immediate life-saving therapeutic interventions (5). In ICU patients with severe COVID-19, endothelial dysfunction has been identified to play a crucial role in the disease progression, thus providing a rationale to monitor bio-ADM® to guide the therapy stabilizing the endothelium (6). Dr. Andreas Bergmann, CEO and founder of SphingoTec commented: “The endothelial function plays a key role in a large number of critical care conditions, such as sepsis, septic shock, acute heart failure, and COVID-19. A distortion in the endothelial function predicts the patient’s progression to a critical stage. Therefore, bio-ADM® screening can identify high-risk patients who require early life-saving therapeutic intervention. With the launch of this rapid test for bio-ADM® on our widely established Nexus IB10 immunoassay platform, we aim to support earlier treatment decisions and improve outcomes of acute care patients.” The IB10 sphingotest® bio-ADM® assay is commercialized in Europe and other regions that accept CE-IVD certification through SphingoTec’s network of distribution partners for the Nexus IB10 platform, together with a broad menu of standard tests for acute and critical care. ### References: (1) Geven (2018): Vascular Effects of Adrenomedullin and the anti-Adrenomedullin Antibody Adrecizumab in Sepsis. Shock. doi: 1097/SHK.0000000000001103 (2) Mebazaa (2018): Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock (AdrenOSS-1) study. doi:10.1186/s13054-018-2243-2 (3) Caironi (2017): Circulating biologically active adrenomedullin (bio-ADM) predicts hemodynamic support requirement and mortality During Sepsis. Chest. doi: 10.1016/j.chest.2017.03.035. (4) ter Maaten (2019): Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure, Eur J Heart Fail. doi:10.1002/ejhf.1437 (5) Lemasle (2020): Bioactive Adrenomedullin, Organ Support Therapies, and Survival in the Critically Ill: Results from the French and European Outcome Registry in ICU Study, Crit. Care. Med, doi: 10.1097/CCM.0000000000004044 (6) Varga (2020): Endothelial cell infection and endotheliitis in COVID-19, Lancet, doi.org/10.1016/S0140-6736(20)30937-5 About SphingoTec SphingoTec GmbH ("SphingoTec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. SphingoTec's proprietary biomarker portfolio includes Bioactive Adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. In addition, SphingoTec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for SphingoTec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by SphingoTec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. About Nexus Dx Inc. and the IB10 Platform Nexus Dx Inc., a wholly-owned subsidiary of SphingoTec, headquartered in San Diego, CA, USA, is a global provider of a near patient testing system and advanced diagnostic solution. The company is improving patient care by providing the medical community with rapid and reliable information at the point of care (POC), delivering patient information when and where it is needed most. The company has invested over $160m to develop and market the IB10 analyzer system which, without the need for sample preparation, automatically separates plasma from whole blood with subsequent reliable and quantitative detection of biomarkers in the plasma by means of antibodies. With a hands-on-time of less than 3 minutes the easy-to-use system provides in only 20 minutes test results for biomarkers that are crucial in the management of critical care patients. The portfolio of IB10 assays includes tests for established critical care parameters such as Procalcitonin, Troponin I, CK-MB, Myoglobin, NT-proBNP, and D-Dimer as well as tests for SphingoTec’s proprietary biomarkers such as DPP3, an assay for Dipeptidyl Peptidase 3, a unique and proprietary biomarker for cardiac depression, Proenkephalin (penKid®), a unique and proprietary biomarker for real-time assessment of kidney function and the IB10 assay for bioactive Adrenomedullin (bio-ADM®), a unique and proprietary biomarker for endothelial function. About bio-ADM® sphingotest® bio-ADM® measures blood levels of bioactive adrenomedullin (bio-ADM®), a hormone maintaining endothelial function. The endothelium contributes to blood pressure and separates blood from the surrounding tissue. Elevated blood levels of bio-ADM® predict blood pressure break down and leaky vessels resulting in oedema. Imbalanced endothelial function is the major cause of shock ultimately resulting in organ dysfunction and death. Early identification of an imbalance in endothelial function allows guidance of vasopressor and diuretic therapy in critically ill patients to improve outcomes. Contact SphingoTec GmbH Ruxandra Lenz Sr. Manager Marketing and Communications Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com
Results of a consensus meeting of clinical experts investigating sphingotec’s diagnostic solutions for acute and critical care in COVID-19. sphingotec’s biomarkers penKid, bio-ADM, and DPP3 previously shown to predict the need for organ support in numerous critical care conditions also have utility in risk stratification of severely ill COVID-19 patients. Hennigsdorf/Berlin, Germany, June 18, 2020 – Diagnostics company SphingoTec GmbH (“sphingotec”) announced today the results of a working group meeting of clinical experts that discussed the utility of sphingotec’s diagnostic solutions for acute and critical care in supporting the triage, diagnosis, and management of severely ill COVID-19 patients. The experts representing European medical centers agreed that novel biomarkers are required for triaging and monitoring of these patients to optimally make use of the available medical resources and improve outcomes. Diagnostic tests for sphingotec’s organ-specific biomarkers for monitoring the endothelial and kidney function are currently investigated on-site in COVID-19 patients in hospitals in Germany, Switzerland, United Kingdom, France and Italy. Serial measurements of sphingotec’s real-time endothelial function biomarker bio-ADM, kidney function biomarker penKid and cardiac depressant factor DPP3 were performed in these centers to investigate their utility in the management of COVID-19 patients. In a working meeting, the investigators discussed their initial findings to develop a consensus on the diagnostic needs for severely ill Covid-19 patients and how sphingotec’s biomarkers can address these needs. Prof. Thorsten Brenner (Essen University Hospital/Essen, Germany) commented: “To identify high risk patients, we need early and specific markers for organ failure to be measured already in the emergency departments. PenKid is a very promising biomarker that we are currently investigating for the triage and the monitoring of organ support therapies of COVID-19 patients with impaired kidney function.” Previously gathered evidence confirmed the central role of bioactive Adrenomedullin and endothelial function in sepsis patients. First feedback on the biomarker bio-ADM in severe COVID-19 describes a strong correlation between high bio-ADM blood levels and the severity of the disease and the need for organ support which can assist in a more accurate and objective risk stratification. Further investigations with DPP3, penKid and bio-ADM are ongoing in countries strongly affected by the COVID-19 pandemic, such as France, Italy and United Kingdom as well as in the United States and Asia. The main objective is to confirm the utility of sphingotec’s biomarkers in the management of COVID-19 patients. Dr. Marlies Ostermann, MD, PhD (Guy’s & St Thomas’ Hospital/London, United Kingdom) commented: “There is an urgent need to assess and monitor organ function of COVID-19 patients in real- time. After a first positive evaluation of these novel biomarkers we have decided to further investigate their use in clinical practice. Especially in the intensive care units, we need biomarkers for monitoring of organ support therapies. This information will also enable us to develop strategies for individualized management of this high-risk group.” The rapidly evolving COVID-19 pandemic has challenged health systems world-wide, with about 5% of patients requiring admission to intensive care units (ICU). Among the main complications that include respiratory, cardiovascular and kidney diseases, an emerging body of evidence shows that endothelial function (1) plays a central role in severe COVID-19 patients. Previous clinical data from more than 22,000 patients demonstrate that high bio-ADM levels independently from inflammation and co-morbidities indicate distortions in the endothelial barrier function, the inner cell sheet of blood vessels. Loss of this barrier function is considered a key driver in the development of hypotension and subsequent septic shock with loss of organ perfusion in sepsis patients (2). According to a Chinese Study in Wuhan, among the non-surviving COVID-19 patients, sepsis was present in all cases (3). Another frequently occurring complication in COVID-19 patients is loss of kidney function. Proenkephalin (penKid) has been demonstrated to be the most accurate surrogate marker for true glomerular filtration rate in patients with renal impairment, without being biased by co-morbidities. DPP3, sphingotec’s novel biomarker for hemodynamic instability and cardiac depression, is a major cause of short-term organ failure when released in an uncontrolled manner into the blood stream. Previously published data (4,5) has shown that high blood levels of DPP3 strongly predict poor outcome in patients with cardiogenic shock. Dr. Andreas Bergmann, founder and CEO of sphingotec said: “To support the critical care community in the management of acute care patients, including COVID-19 patients, we have made available rapid tests for our biomarkers penKid®, bio-ADM® and DPP3 on our whole-blood Nexus IB10 point-of-care platform.” The novel biomarker tests complement a wide-range of assays for acute care settings that are already available on sphingotec’s widely used Nexus IB10 point-of-care platform that can be flexibly deployed in laboratory as well as near-patient settings such as emergency departments and intensive care units. References: (1) Varga et al (2020): Endothelial cell infection and endothelitis in COVID-19, DOI:https://doi.org/10.1016/S0140-6736(20)30937-5 (2) Mebazaa et al (2018): Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) study, Crit Care, doi: 10.1186/s13054-018-2243-2 (3) Zhou et at (2020): Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Doi: doi.org/10.1016/S0140-6736(20)30566-3 (4) Takagi (2019) Circulating dipeptidyl-peptidase 3 and alteration in hemodynamics in cardiogenic shock: Results from the OptimaCC Trial, European Journal of Heart Failure, doi: 10.1002/ejhf.1600 (5) Deniau (2019) Circulating dipeptidyl peptidase-3 is a myocardial depressant factor: DPP3 inhibition rapidly and sustainably improves hemodynamics, European Journal of Heart Failure, doi: 10.1002/ejhf.1601 About sphingotec SphingoTec GmbH ("sphingotec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. sphingotec's proprietary biomarker portfolio includes Bioactive Adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. In addition, sphingotec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for sphingotec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by sphingotec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. About bio-ADM® sphingotest® bio-ADM® measures blood levels of bioactive adrenomedullin (bio-ADM®), a hormone maintaining endothelial function. The endothelium contributes to blood pressure and separates blood from the surrounding tissue. Elevated blood levels of bio-ADM® predict blood pressure break down and leaky vessels resulting in oedema. Imbalanced endothelial function is the major cause of shock ultimately resulting in organ dysfunction and death. Early identification of an imbalance in endothelial function allows guidance of vasopressor and diuretic therapy in critically ill patients to improve outcomes. About DPP3 IB10 sphingotest® DPP3 is a rapid point-of-care (POC) immunoassay for the in vitro quantitative determination of Dipeptidyl peptidase 3 an active enzyme which, when released into the blood, inactivates angiotensin II, a hormone that is important for the heart function. This inactivation leads to hemodynamic instability and consequently cardiac depression. The DPP3 release is a newly identified disease mechanism explaining short-term organ failure in critically ill patients. Early identification of DPP3 release may allow better patient stratification and earlier therapy escalation to improve outcomes. About penKid® sphingotest® penKid® measures proenkephalin (penKid®), a stable fragment of the kidney stimulating hormone enkephalin. penKid® has been demonstrated to be a real-time surrogate biomarker for glomerular filtration rate, the gold standard to assess renal function. Measuring penKid® blood concentrations allows for timely information on kidney function in critically ill patients. Early assessment of worsening and improving of renal function on intensive care units and in emergency departments allows adjustment of nephrotoxic drug administration and the initiation of kidney-protective strategies to prevent acute kidney injury and thereby improve outcomes. Contact SphingoTec GmbH Ruxandra Lenz Sr. Manager Marketing and Communications Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com
DPP3 is a highly dynamic marker for predicting and monitoring cardiac depression in burn patients High DPP3 blood concentrations are indicating multiple organ failure and poor outcomes sphingotec commercializes a rapid CE-IVD test for DPP3 on its proprietary point-of-care platform Nexus IB10 Hennigsdorf/Berlin, Germany, May 14, 2020 – Diagnostics company SphingoTec GmbH (“sphingotec”) and 4TEEN4 Pharmaceuticals GmbH (“4TEEN4”) announced today the publication of new data showing that high blood levels of Dipeptidyl Peptidase 3 (DPP3) are indicating upcoming multiple organ failure and mortality risk in burn patients. DPP3 is a proprietary biomarker of 4TEEN4 for hemodynamic instability and cardiac depression. sphingotec has in-licensed global rights to develop and commercialize in vitro diagnostic (IVD) tests for the DPP3 biomarker from 4TEEN4 and made it available on its proprietary Nexus IB10 point-of-care platform. The results from the recent study1 provide evidence that DPP3 blood concentrations in severely ill burn patients are indicating fatal outcomes. The data also shows that high DPP3 concentration in the blood is linked to circulatory failure, cardiac depression, and acute kidney injury. Decreasing DPP3 levels in the blood, on the other hand, indicate a substantially reduced risk of mortality. According to a newly identified disease mechanism, the release of the cardiac depressant factor DPP3 into the bloodstream is a major cause of short-term organ failure: DPP3 is an enzyme that is present in many cell types and normally plays an important role in the recycling of cellular proteins. When massive uncontrolled cell death occurs, like in the case of burn patients, DPP3 is released into the bloodstream where it degrades angiotensin II, a peptide hormone controlling the heart function. Unphysiologically low levels of angiotensin II rapidly lead to cardiac depression and ultimately organ failure. Previously published data2,3 provided evidence that poor outcome of patients with severe heart failure or cardiogenic shock are caused by DPP3. Furthermore, the causal role of DPP3 in cardiac depression could be reproduced in several model systems. “The new data adds to the growing body of evidence that places the cardiac depressant factor DPP3 in strong connection with short-term organ failure and high mortality in critical care settings. We have already started a collaboration with the critical care community to provide our fully automated DPP3 point-of-care test to support the management of acute care patients,” said Dr. Andreas Bergmann, CEO and founder of sphingotec. The IVD test for DPP3 is commercialized under the brand name IB10 sphingotest® DPP3 and is designed and validated for use in conjunction with sphingotec’s fully automated Nexus IB10 whole blood point-of-care platform, delivering results within 20 minutes. This new test complements a wide-range of assays for acute care settings that are already available on this widely used point-of-care platform that can be flexibly deployed in laboratory as well as near-patient settings such as emergency departments and intensive care units. References (1) Dépret (2020) Circulating dipeptidyl peptidase-3 at admission is associated with circulatory failure, acute kidney injury and death in severely ill burn patients, Critical Care. doi: 10.1186/s13054-020-02888-5. (2) Takagi (2019) Circulating dipeptidyl-peptidase 3 and alteration in hemodynamics in cardiogenic shock: Results from the OptimaCC Trial, European Journal of Heart Failure, doi: 10.1002/ejhf.1600 (3) Deniau (2019) Circulating dipeptidyl peptidase-3 is a myocardial depressant factor: DPP3 inhibition rapidly and sustainably improves hemodynamics, European Journal of Heart Failure, doi: 10.1002/ejhf.1601 ### About sphingotec SphingoTec GmbH ("sphingotec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. sphingotec's proprietary biomarker portfolio includes Bioactive Adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. In addition, sphingotec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for sphingotec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by sphingotec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of established and commonly used tests for acute and critical care. About 4TEEN4 4TEEN4 Pharmaceuticals GmbH (“4TEEN4”) is a biopharmaceutical company developing Procizumab, a humanized antibody targeting human Dipeptidyl Peptidase 3 (DPP3) for the treatment of critically ill patients suffering from cardiac depression and multiple organ failure. 4TEEN4 licenses its proprietary biomarker DPP3 to make it available for diagnostic use in indications as acute heart failure, cardiogenic shock, septic shock and other critical care conditions. The company was established in 2013 in Hennigsdorf near Berlin, Germany, by Dr. Andreas Bergmann, CEO of 4TEEN4, as part of his Medicine4Future Initiative. About DPP3 IB10 sphingotest® DPP3 is a rapid point-of-care (POC) immunoassay for the in vitro quantitative determination of Dipeptidyl peptidase 3 an active enzyme which, when released into the blood, inactivates angiotensin II, a hormone that is important for the heart function. This inactivation leads to hemodynamic instability and consequently cardiac depression. The DPP3 release is a newly identified disease mechanism explaining short-term organ failure in critically ill patients. Early identification of DPP3 release may allow better patient stratification and earlier therapy escalation to improve outcomes. Contact SphingoTec GmbH Ruxandra Lenz Sr. Manager Marketing and Communications Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com

Hennigsdorf/Berlin, Germany, April 16, 2020 - Diagnostics company SphingoTec GmbH ("sphingotec", Hennigsdorf, Germany) today announced that it has appointed Dr. Achim Plum as Managing Director and Chief Commercial Officer (CCO) of the company. As CCO, Dr. Plum will focus on driving the global commercialization of sphingotec’s IVD solutions for novel biomarkers for acute and critical care settings and their adoption on multiple IVD instrument platforms, such as sphingotec’s proprietary point-of-care platform Nexus IB10 for rapid testing at the point of need. “With sphingotec advancing to an end-to-end diagnostic solutions provider with international presence, I am delighted to welcome Dr. Plum to our leadership team,” said Andreas Bergmann, Founder, Managing Director and CEO of sphingotec. “Dr. Plum’s extensive business experience, as well as his broad management expertise in the diagnostics industry, will strengthen our organization and help scale it to an integrated global commercial operation providing unique diagnostic solutions for highly unmet needs in acute and critical care.” Dr. Achim Plum has more than 18 years of management and commercial experience in the diagnostics industry with a focus on innovative diagnostics solutions, biomarker development and commercialization, and personalized medicine. As CCO of sphingotec he is in charge of all business and commercial operations of the company. After having worked with sphingotec as a business advisor since May 2019, he in April 2020 joined sphingotec full-time from a position as Executive Board Member and Chief Business Officer of the Curetis Group, developing and commercializing molecular diagnostic solutions for infectious diseases. Before Curetis, he held senior management positions at Siemens Healthcare where he was responsible for global Diagnostic and BioScience Technology & Innovation. Prior to Siemens, Dr. Plum worked for eight years for the cancer molecular diagnostics company Epigenomics, lastly as SVP Business & Strategy, the molecular diagnostics that developed and commercializes the first FDA-cleared liquid biopsy colorectal cancer screening test. Dr. Plum studied Genetics, Cell Biology, and Biochemistry in Bonn, Germany, and Norwich UK. He obtained his Ph.D. in Molecular Genetics from the University of Bonn in 1999. Dr. Achim Plum commented: “I am very excited to now fully join the sphingotec team. As an advisor to the company since May last year I got increasingly impressed by sphingotec’s biomarker innovations and the enormous body of evidence and network of key opinion leaders supporting their unique utility in acute and critical care. With our Nexus IB10 point-of-care testing platform, we are well-positioned to rapidly implement our solutions in hospitals worldwide and significantly improving the outcomes of critically ill patients by providing clinicians with actionable and timely information.” Sphingotec’s portfolio proprietary tests for the real-time assessment and monitoring of endothelial function (IB10 sphingotest® bio-ADM®), kidney function (IB10 sphingotest® penKid®), and cardiac depression (IB10 sphingotest® DPP3) is made available on its proprietary immunoassay point-of-care platform, Nexus IB10, that also features a broad menu of established routine tests tailored to point-of-care use. For the broad availability of its diagnostic solutions on multiple platforms for central laboratories, sphingotec pursues a licensing strategy with global players in the diagnostics industry. About sphingotec sphingotec GmbH ("sphingotec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. sphingotec's proprietary biomarker portfolio includes bioactive adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardiac depression. In addition, sphingotec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for sphingotec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by sphingotec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of IB10 tests for established biomarkers for acute and critical care. Contact SphingoTec GmbH Ruxandra Lenz Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com

New study data show that monitoring blood levels of sphingotec’s endothelial function biomarker bio-ADM® on top of guideline parameter lactate improves risk stratification of sepsis patients admitted to intensive care units Monitoring of both, lactate and bio-ADM®, in sepsis patients allows early identification of patients that require immediate intervention at admission to the ICU. Sphingotec is set to launch a point-of-care bio-ADM® assay running on its proprietary automated Nexus IB10 instrument in mid-2020. Hennigsdorf/Berlin, Germany, March 5, 2020 - Diagnostics company SphingoTec GmbH ("sphingotec", Hennigsdorf Germany) today reported on new data on the utility of endothelial function biomarker bioactive Adrenomedullin (bio-ADM®). The data show that bio-ADM® allows identification of sepsis patients who are at high risk of fatal outcomes despite low or decreasing levels of the routinely monitored parameter lactate. Lactate, a parameter that identifies reduced blood oxygenation of tissue, is routinely used as a reference in the diagnosis of septic shock. However, lactate is rather unspecific to sepsis and insensitive. This limitation can be overcome by monitoring, in addition to lactate, the blood levels of bio-ADM®, a biomarker that can reliably detect blood vessel leakage, one of the main causes of septic shock. According to recent findings published in Critical Care[1], data from over 500 sepsis patients enrolled in the AdrenOSS-1 study demonstrate the added value of bio-ADM® to lactate monitoring. The AdrenOSS-1 study investigators could show that even though normalizing lactate levels indicate a significantly decreased risk of mortality, an additional measurement of bio-ADM® blood levels can help identify those patients that are still at risk of fatal outcomes despite their lower lactate levels. Among septic patients with decreasing lactate, high bio-ADM® levels identified patients who had a 4-time higher mortality risk than patients with low bio-ADM levels. According to the authors of the study, measurement of bio-ADM® on top of lactate may help refine risk stratification and thus guide resuscitation during sepsis. Lactate has been used for more than 30 years to monitor organ hypoperfusion in sepsis patients. However, lactate blood levels are influenced by many other physiological and pathological processes. Clinical data from more than 22,000 patients demonstrate that high bio-ADM® levels independently from inflammation and co-morbidities indicate distortions in the barrier function of the inner cell sheet of blood vessels, the endothelium. Loss of this barrier function is considered a key driver in the development of hypotension and eventually septic shock with loss of organ perfusion in sepsis patients[2]. According to sphingotec’s research, bio-ADM® can explain about 60% of the fatal outcomes in sepsis. “Our biomarker bio-ADM® can reliably support acute care physicians in identifying high-risk sepsis patients” said Dr. Andreas Bergmann, founder and CEO of sphingotec. “We are set to launch the fully automated CE-IVD-marked point-of-care bio-ADM® assay on our widely established Nexus IB10 immunoassay instrument by mid-2020. We are convinced that this rapid test for bio-ADM® will support earlier treatment decisions and thereby will assist clinical decisions that may improve the outcomes of patients at ICUs and emergency departments.” References 1. Benjamin G. Chousterman et al (2020): Added value of serial bio-adrenomedullin measurement in addition to lactate for the prognosis of septic patients admitted to ICU,. doi:10.1186/s13054-020-2794-x 2. Mebazaa et al (2018): Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) study, Crit Care, doi: 10.1186/s13054-018-2243-2

Bioactive Adrenomedullin is a biomarker for endothelial dysfunction and allows the prediction of septic shock as elevated blood levels of bio-ADM® predict blood pressure break down and blood vessel leakage resulting in edema AdrenOSS-2 Phase II trial shows that modulating the Adrenomedullin plasma level with the therapeutic antibody Adrecizumab demonstrates an improvement of survival in patients with septic shock Increased bio-ADM® levels were used as inclusion criteria in the AdrenOSS-2 Phase II trial to identify patients with endothelial dysfunction sphingotec to launch IB10 sphingotest® bio-ADM® a rapid immunoassay for bioactive Adrenomedullin on its Nexus IB10 point-of-care platform mid 2020 Hennigsdorf/Berlin, Germany, February 24, 2020 - Diagnostics company SphingoTec GmbH ("sphingotec", Hennigsdorf, Germany) today announced that data from the AdrenOSS-2 study indicate a modulating role of bioactive Adrenomedullin in septic shock. Topline results of the AdrenOSS-2 Phase II trial released on February 21, 2020 by its sponsor Adrenomed AG (Adrenomed) showed an increase in survival for patients with septic shock when treated with Adrecizumab. Adrecizumab targets bioactive Adrenomedullin and modulates endothelial function. Septic shock is the most severe form of sepsis, a medical emergency with high mortality. In the trial, blood levels of bioactive Adrenomedullin were measured with sphingotec’s quantitative bio-ADM® immunoassay as inclusion criteria. Bioactive Adrenomedullin has been previously validated in over 22,000 patients as an endothelial function biomarker whose detection in the blood provides dynamic information on the patients’ progression in sepsis. High or rising blood levels of bioactive Adrenomedullin indicate a disbalance in the endothelial function leading to edema and shock while decreasing levels have been linked to improved patient outcomes. Based on this evidence, bioactive Adrenomedullin is not only a valid biomarker for endothelial function but was also qualified as a biotarget and led to the subsequent development of Adrecizumab, a therapeutic antibody to treat septic shock. In the AdrenOSS-2 Phase II trial, which was designed to evaluate the safety, tolerability, and efficacy of Adrecizumab, increased bio-ADM® levels were used as an inclusion criterion to select those patients with septic shock, which have an endothelial dysfunction. The trial enrolled a total of 301 patients with septic shock and was carried out in multiple clinical trial centers in Belgium, France, Germany, and The Netherlands. In this proof-of-concept trial, patients received Adrecizumab or placebo on top of standard of care treatment. The study achieved its primary endpoint: Adrecizumab demonstrated a favorable safety profile and was well tolerated. In addition, a lower all-cause mortality for Adrecizumab-treated patients was observed when compared to placebo. AdrenOSS-2’ principal investigator Pierre-François Laterre commented: “Using bioactive Adrenomedullin blood levels as inclusion criteria allowed us to select the patients who have endothelial dysfunction. Measuring bioactive Adrenomedullin in a routine situation, furthermore allowed us to better understand the utility of this biomarker as a diagnostic tool as we saw that bioactive Adrenomedullin gave information on top of clinical standard parameters to assess patients’ severity. We will now go ahead to further investigate use-cases of bioactive Adrenomedullin as a biomarker in the clinical routine.” Dr. Andreas Bergmann, founder and CEO of sphingotec commented: “Following our approach of diving deep into the biology of the disease, we have developed biomarkers that support clinicians in identifying the root causes of progression in sepsis. While the current trial indicates that using bioactive Adrenomedullin as a biomarker can help identifying those patients who could benefit in the future from Adrecizumab, the utility of this biomarker expands far beyond this and supports already today the early clinical decision making and monitoring of treatment success in sepsis.” To support the critical care community in the timely assessment of endothelial function in sepsis and other acute and critical care conditions, sphingotec will launch IB10 sphingotest® bio-ADM®, a rapid test for bioactive Adrenomedullin that will be deployed on the company’s proprietary point-of-care platform, the Nexus IB10 immunoassay analyzer. ### About sphingotec SphingoTec GmbH ("sphingotec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. sphingotec's proprietary biomarker portfolio includes Bioactive Adrenomedullin (bioADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardio-renal pathway disruptions leading to acute organ dysfunction. In addition, sphingotec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for sphingotec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by sphingotec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of IB10 tests for established biomarkers for acute and critical care. About Nexus Dx Inc. and the IB10 Platform Nexus Dx Inc., a wholly-owned subsidiary of sphingotec, headquartered in San Diego, CA, USA, is a global provider of a near patient testing system and advanced diagnostic solution. The company is improving patient care by providing the medical community with rapid and reliable information at the point of care (POC), delivering patient information when and where it is needed most. The company has invested over $160m to develop and market the IB10 analyzer system which, without the need for sample preparation, automatically separates plasma from whole blood with subsequent reliable and quantitative detection of biomarkers in the plasma by means of antibodies. With a hands-on-time of less than 3 minutes the easy-to-use system provides in only 20 minutes test results for biomarkers that are crucial in the management of critical care patients. The portfolio of IB10 assays includes tests for established critical care parameters such as Procalcitonin, Troponin I, CK-MB, Myoglobin, NTproBNP, and D-Dimer as well as tests for sphingotec’s proprietary biomarkers such as DPP3, an assay for Dipeptidyl Peptidase 3, a unique and proprietary biomarker for cardio-renal pathway disruptions leading to acute organ dysfunction, and Proenkephalin (penKid®), a unique and proprietary biomarker for real-time assessment of kidney function. An IB10 assay for bioactive Adrenomedullin (bio-ADM®), a unique and proprietary biomarker for endothelial function is expected to be launched later in 2020. About bio-ADM® sphingotest® bio-ADM® measures blood levels of bioactive adrenomedullin (bio-ADM®), a hormone maintaining endothelial function. The endothelium contributes to blood pressure and separates blood from the surrounding tissue. Elevated blood levels of bio-ADM® predict blood pressure break down and leaky vessels resulting in oedema. Imbalanced endothelial function is the major cause of shock ultimately resulting in organ dysfunction and death. Early identification of an imbalance in endothelial function allows guidance of vasopressor and diuretic therapy in critically ill patients to improve outcomes. About Adrecizumab Adrenomed’s first-in-class drug candidate Adrecizumab targets adrenomedullin (ADM) to rescue endothelial barrier function (= vascular integrity). Binding of the monoclonal antibody Adrecizumab to ADM in the blood traps and stabilizes the peptidehormone resulting in increased ADM concentrations within the blood vessels. The complex of ADM and Adrecizumab in the blood is still active. This way, Adrecizumab treatment boosts Adrenomedullin’s protective effects on the endothelial barrier. www.adrenomed.com Contact SphingoTec GmbH Ruxandra Lenz Neuendorfstr. 15 A 16761 Hennigsdorf Germany Tel. +49-3302-20565-0 press@sphingotec.de www.sphingotec.com
Numerous talks and posters on sphingotec’s acute care biomarkers in the scientific program of the conference sphingotec to host symposium “Relevant Pathways, Novel Diagnostic and Therapeutic Approaches in Acute Organ Dysfunction” Hennigsdorf, Germany, February 21, 2020 - SphingoTec GmbH (“sphingotec”) today announced that novel data on its acute care biomarkers Proenkephalin (penKid®) and Dipeptidyl Peptidase 3 (DPP3) will be presented at the 25th International Conference on Advances in Critical Care Nephrology (AKI & CRRT 2020) taking place in San Diego, CA, USA, on February 24-27, 2020. AKI & CCRT 2020 Presentations on penKid® and DPP3 In the scientific program of the conference, clinical researchers will present the latest results from studies investigating these two critical care biomarkers and their utility in diagnosing kidney injury. In a session on translating biomarker discoveries to clinical care, Peter Pickkers (Radboud University | RU · Department of Intensive Care, Nijmegen, The Netherlands) will present data demonstrating that sphingotec’s kidney function marker penKid® is a reliable surrogate for assessing true glomerular filtration rate (true GFR) in patients with renal insufficiency. Previous studies demonstrated that penKid® is a real-time kidney function biomarker, which is independent of inflammation and other co-morbidities. In another session, Matthieu Legrand (University of California, San Francisco | UCSF · Department of Anesthesia and Perioperative Care, USA) will provide evidence showing that sphingotec’s biomarker DPP3 is one cause for short-term kidney dysfunction and is highly associated with the development of acute kidney injury (AKI) in patients with severe burns. Being at the core of a newly discovered disease mechanism, DPP3 has been shown to play a causal role in short-term organ dysfunction when released into the bloodstream upon massive cell death in several acute and critical care conditions. Sphingotec Symposium at AKI & CCRT 2020 In a symposium organized by sphingotec titled “Relevant Pathways, Novel Diagnostic and Therapeutic Approaches in Acute Organ Dysfunction” leading clinical researchers will further discuss pathways leading to acute organ dysfunctions in critically ill patients as well as a novel diagnostic solution to assess the involvement of such pathways in acute and critical care conditions. Beyond providing an overview of novel biomarkers for monitoring kidney function, endothelial function, and DPP3-mediated heart and kidney dysfunction, the speakers will also share findings that are qualifying these biomarkers as biotargets. ### Oral Presentations at AKI & CRRT 2020 Session: Bench to Bedside: Translating Discoveries to Clinical Care, February 24, 2020 “Proenkephalin: Is it the New GFR Marker?” presented by Peter Pickkers (Radboud University | RU · Department of Intensive Care) Session: Acute Kidney Injury (AKI): Pathophysiology, February 25, 2020 “DPP3 in Critical Illness: Another Piece of the Puzzle” presented by Matthieu Legrand (University of California, San Francisco | UCSF · Department of Anesthesia and Perioperative Care) Poster Presentation at AKI & CRRT 2020 Title: Proenkephalin, a Novel Biomarker for Kidney Function, is Earlier in Detecting Acute Kidney Injury Compared to Creatinine. Authors R Beunders, M Meekes, J Struck, P Pickkers Title: Proenkephalin Predicts Renal Dysfunction, Organ Failures, Renal Replacement Therapy, and Mortality in Sepsis Authors H Kim, M Hur, J Struck, A Bergmann, S Di Somma Title: penKid Proenkephalin A 119–159 effectively predicts Acute Kidney Injury, Multiple Organ Failure, Mortality particularly among septic patients at the ER with seemingly intact renal function Authors M Rosenqvist, K Bronton, O Hartmann, A Bergmann, J Struck & O Melander About sphingotec SphingoTec GmbH ("sphingotec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. sphingotec's proprietary biomarker portfolio includes Bioactive Adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardio-renal pathway disruptions leading to acute organ dysfunction. In addition, sphingotec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for sphingotec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by sphingotec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of IB10 tests for established biomarkers for acute and critical care.

In-depth method comparison by Dutch group shows that sphingotec's proprietary kidney function biomarker penKid® is currently the most accurate surrogate marker for true glomerular filtration rate in patients with renal impairment Data by PRONOBURNS group show that penKid® accurately predicts acute kidney injury (AKI) in patients with burns, adding benefit on top of standard testing Since January 2020, an automated CE-marked IVD penKid® assay developed for sphingotec's proprietary point-of-care platform Nexus IB10 is available for use in critical care settings Hennigsdorf/Berlin, Germany, February 19, 2020 - Diagnostics company SphingoTec GmbH ("sphingotec", Hennigsdorf Germany) today announced the publication of two studies demonstrating that its kidney function biomarker Proenkephalin (penKid®) is the most accurate surrogate for assessing true glomerular filtration rate (true GFR) and is reliably predictive for acute kidney injury (AKI) in patients with severe burns. Although AKI is a major complication in critically ill patients, current diagnostic standard methods do not properly and timely diagnose impaired renal function. The two recently published studies add to the rapidly growing body of evidence suggesting that penKid® can address this highly unmet diagnostic need. In Shock[1], a team headed by Prof. Peter Pickkers from Radboud UMC (Nijmegen, The Netherlands) confirmed by in-depth diagnostic method comparison in patients with impaired kidney function that penKid® levels properly reflect kidney function. While today’s standard of care uses estimations of the glomerular filtration rate (eGFR) to assess renal impairment, the current study shows that these methods overestimate true GFR with over 30%. The published findings demonstrate that penKid® can add value by properly reflecting true GFR that can otherwise only be measured using in vivo clearance of iohexol, an invasive method too laborious and time-consuming for clinical routine use. Another study published in the Journal of Burns[2] reports for the first time that high penKid® plasma levels at admission to the intensive care unit (ICU) were associated with the risk for developing AKI in patients with severe burns, where mortality rates range from 30-70%.The new data suggest, that the current renal function- and AKI standard markers should be complemented with penKid® values for accurately quantifying the kidney function in critically ill patients. Dr. Andreas Bergmann, CEO of sphingotec, commented: “penKid® is an early renal function biomarker that is not biased by co-morbidities while reflecting true GFR. penKid® has been tested for the first time to identify ICU patients with severe burns that need rapid and aggressive intervention to prevent mortality caused by AKI.” To support timely treatment decisions that are likely to improve patient management in critical care patients, sphingotec launched a fully automated CE-IVD-marked penKid® assay on its Nexus IB10 platform in January 2020. This new test complements a wide-range of assays for acute care settings that are already available on this widely used point-of-care platform that can be flexibly deployed in near-patient as well as laboratory settings. ### References 1. Beunders, R. et al., Proenkephalin compared to conventional methods to assess kidney function in critically ill sepsis patients, Shock,, doi:10.1097/SHK.0000000000001510 2. Depret, F. et al, PenKid measurement at admission is associated with outcome in severely ill burn patients, J.Burns, doi: doi.org/10.1016/j.burns.2020.01.002 About sphingotec SphingoTec GmbH ("sphingotec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. sphingotec's proprietary biomarker portfolio includes Bioactive Adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardio-renal pathway disruptions leading to acute organ dysfunction. In addition, sphingotec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for sphingotec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by sphingotec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of IB10 tests for established biomarkers for acute and critical care. About penKid® sphingotest® penKid® measures proenkephalin (penKid®), a stable fragment of the kidney stimulating hormone enkephalin. penKid® has been demonstrated to be a real-time surrogate biomarker for glomerular filtration rate, the gold standard to assess renal function. Measuring penKid® blood concentrations allows for timely information on kidney function in critically ill patients. Early assessment of worsening and improving of renal function on intensive care units and in emergency departments allows adjustment of nephrotoxic drug administration and the initiation of kidney-protective strategies to prevent acute kidney injury and thereby improve outcomes. About Nexus Dx Inc. and the IB10 Platform Nexus Dx Inc., a wholly-owned subsidiary of sphingotec, headquartered in San Diego, CA, USA, is a global provider of a near patient testing system and advanced diagnostic solution. The company is improving patient care by providing the medical community with rapid and reliable information at the point of care (POC), delivering patient information when and where it is needed most. The company has invested over $160m to develop and market the IB10 analyzer system which, without the need for sample preparation, automatically separates plasma from whole blood with subsequent reliable and quantitative detection of biomarkers in the plasma by means of antibodies. With a hands-on-time of less than 3 minutes the easy-to-use system provides in only 20 minutes test results for biomarkers that are crucial in the management of critical care patients. The portfolio of IB10 assays includes tests for established critical care parameters such as Procalcitonin, Troponin I, CK-MB, Myoglobin, NT-proBNP, and D-Dimer as well as tests for sphingotec’s proprietary biomarkers such as DPP3, an assay for Dipeptidyl Peptidase 3, a unique and proprietary biomarker for cardio-renal pathway disruptions leading to acute organ dysfunction, and Proenkephalin (penKid®), a unique and proprietary biomarker for real-time assessment of kidney function. An IB10 assay for bioactive Adrenomedullin (bio-ADM®), a unique and proprietary biomarker for endothelial function is expected to be launched later in 2020.

Norderstedt and Hennigsdorf, Germany, January 14, 2020 – Hitado GmbH (Hitado), a Sysmex point-of-care subsidiary, and SphingoTec GmbH (sphingotec) have signed an exclusive distribution agreement for the commercialisation of the Nexus IB10 diagnostic platform by Hitado in Germany. Further, Sysmex Suisse AG will commercialise the platform in Switzerland. The Nexus IB10 platform is a fully automated rapid immunoassay point-of-care platform, that provides accurate test results within only 20 minutes and features a broad menu of tests relevant in near-patient settings, e.g. cardiovascular parameters such as troponin I, NT-proBNP, D-dimer, CK-MB and myoglobin. Further novel and proprietary tests that are developed by sphingotec and provide solutions for highly unmet diagnostic needs in sepsis, acute heart failure and acute kidney injury will complement the existing menu of IB10 test making the platform a unique solution for acute and critical care. The CE-IVD-marked system meets the requirements of near-patient testing and can be flexibly deployed in laboratories, emergency departments, intensive care units, and doctors’ offices. Regarding this new distribution agreement, Hitado’s CEO André Michel commented, “We are delighted to be appointed as the exclusive distribution partner for Nexus IB10 in Germany and Switzerland. This product is a strategic fit with our existing product portfolio and will separate us from our competitors, providing customers with a comprehensive package of patient-near testing devices.” Andreas Bergmann, CEO of sphingotec, echoes those sentiments. He noted, “We are very much looking forward to our collaboration with Hitado and Sysmex who have been very successful in promoting the IB10 platform in the past. We also believe that they are excellent strategic partners for providing rapid and novel solutions needed by clinicians in managing critically ill patients and will support us in our long-term strategy to improve patient outcomes in acute and critical care conditions.” The Nexus IB10 platform was originally developed and commercialised by Samsung under the brand name Samsung LabgeoIB10. Following the acquisition of Samsung’s subsidiary Nexus Dx Inc. (San Diego, CA, USA), the manufacturer of the IB10 platform, by sphingotec in 2018, Nexus IB10 is now exclusively represented by sphingotec and made available through sphingotec’s global network of distribution partners. To discover more about the Nexus IB10 and sphingotec’s portfolio of diagnostic solutions for acute and critical care, please visit sphingotec.com. For more information on the Hitado point-of-care portfolio, please visit hitado.de. About Hitado GmbH Hitado was founded 44 years ago as a provider of diagnostic solutions for hospitals and laboratories and is the leader provider of near-patient diagnostics in Germany. Today, they are a part of the Sysmex Group, a global leader in in-vitro diagnostics. Their portfolio includes highly specific rapid tests for diverse applications including cancer screening, cardiac diagnostics, pregnancy, infection and metabolic diagnostics to specialised point-of-care testing systems. To discover more about Hitado, visit hitado.de. About sphingotec SphingoTec GmbH ("sphingotec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in-vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. sphingotec's proprietary biomarker portfolio includes bioactive adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardio-renal pathway disruptions leading to acute organ dysfunction. In addition, sphingotec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for sphingotec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by sphingotec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of IB10 tests for established biomarkers for acute and critical care.
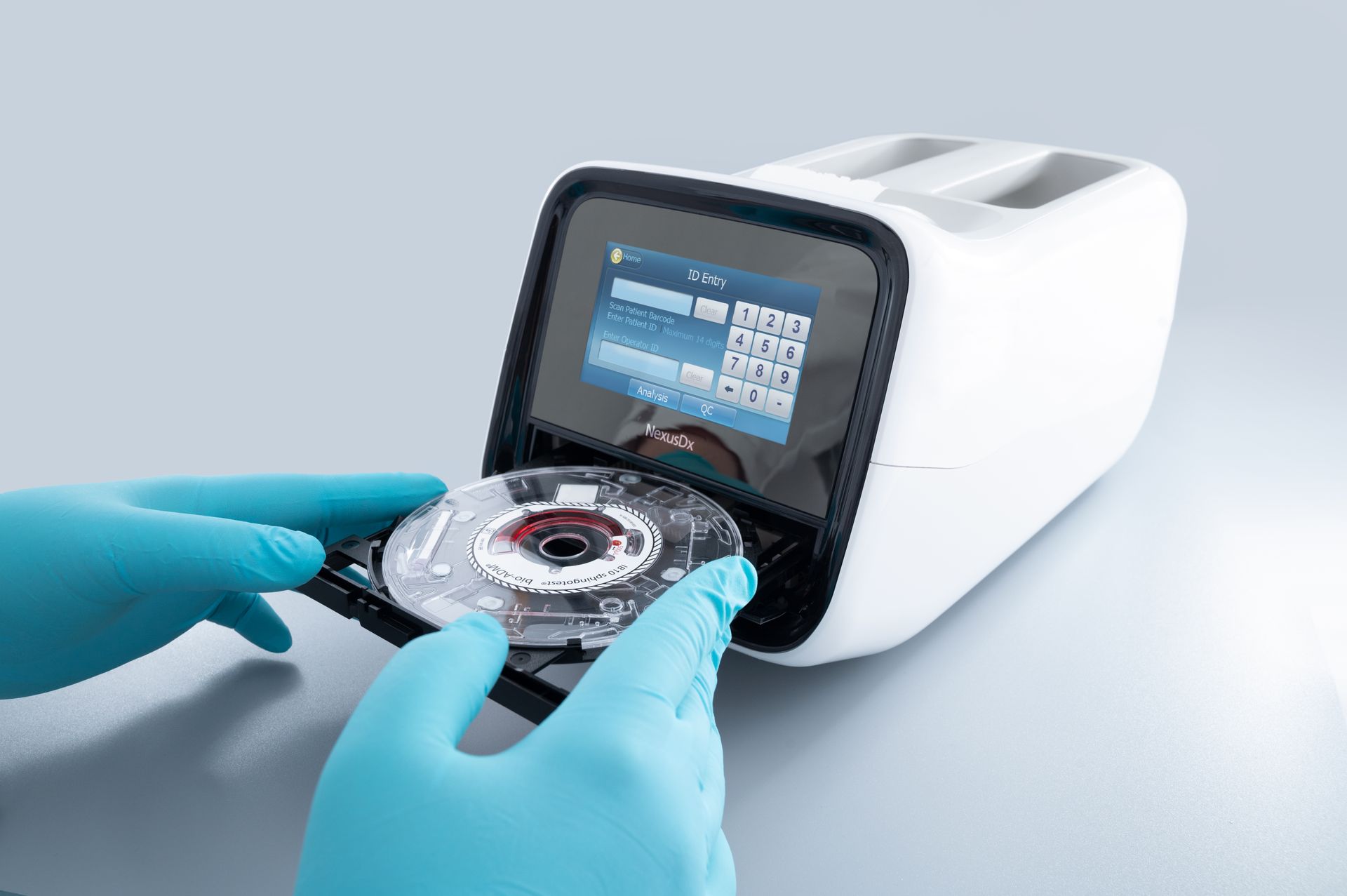
sphingotec launches a CE-marked point-of-care IVD assay for Proenkephalin (penKid®), the company‘s proprietary biomarker for real-time assessment of kidney function in critical care settings IB10 sphingotest® penKid® runs on sphingotec's automated Nexus IB10 point-of-care platform and quantitatively measures levels of penKid® directly in whole blood samples penKid® is a functional kidney biomarker reflecting the actual glomerular filtration rate and thus allows monitoring of renal function in real-time penKid® enables earlier prediction and more precise monitoring of acute kidney injury than the current standard-of-care Hennigsdorf/Berlin, Germany, January 13, 2020 - Diagnostics company SphingoTec GmbH ("sphingotec", Hennigsdorf near Berlin, Germany) today announced the launch of its IB10 sphingotest® penKid®, a CE-IVD-marked point-of-care test for Proenkephalin (penKid®), the biomarker that allows real-time assessment of kidney function with a simple blood test. The test is made available on the company’s rapid point-of-care platform Nexus IB10 that uses whole blood samples without any pre-processing, requires less than three minutes hands-on-time, delivers test results after 20 minutes and can be flexibly deployed in laboratories or near-patient settings such as intensive care units (ICUs) and Emergency Departments (EDs). In studies on more than 30,000 patients admitted to ICUs and EDs, penKid® has been demonstrated to be a real-time surrogate biomarker for glomerular filtration rate[1], the gold standard to assess kidney function. In contrast to other markers, penKid® is an early predictor of worsening and improving kidney function independently from co-morbidities and inflammation[2,3]. penKid® as a functional kidney biomarker has been demonstrated to provide significant clinical utility in numerous critical care settings: – penKid® blood levels at admission to ICUs and EDs can identify patients at a high risk to develop acute kidney injury (AKI) as elevated blood levels of penKid® indicate kidney dysfunction in critically ill patients much earlier than the current standard of care4. – penKid® blood levels during hospitalization can be used for real-time monitoring of kidney function. In critical care conditions (such as sepsis, acute heart failure) or after the administration of nephrotoxic drugs, which trigger kidney damage, elevated blood levels of penKid® reflect compromised kidney function. The normalization of kidney function is rapidly mirrored by decreasing levels of penKid®. Hence, penKid® allows for individualized dosing of medicines with nephrotoxic side-effects. – penKid® blood levels at discharge identify patients who have low levels of this biomarker that can be safely discharged from the hospital while elevated biomarker levels identify patients with sublinical AKI5 that require further attention. Worldwide AKI is affecting 13 million patients and puts other 26 million at risk. In the ICUs, 1 out of 3 patients will develop AKI6. Dr. Andreas Bergmann, CEO and founder of sphingotec commented: "Testing and monitoring of blood levels of Proenkephalin with our IB10 sphingotest® penKid® allows not only for the stratification of patients at risk for acute kidney injury at admission to the hospital but also facilitates the monitoring of kidney function in real-time throughout the entire patient care cycle. We strongly believe that making penKid® testing available on a rapid point-of-care platform has a unique potential to enable timely therapy decision by physicians at ICUs and EDs and thereby improve patient outcomes.” The IB10 sphingotest® penKid® will be made available at the end of January 2020 to the critical care community for further clinical evaluation. The assay is commercialized in Europe and other regions that accept CE-IVD certification through sphingotec’s network of distribution partners for the Nexus IB10 platform, together with a broad menu of standard tests for acute and critical care. ### References 1. Donato L. et al., (2018): Analytical performance of an immunoassay to measure proenkephalin, Clin Biochem., doi: 10.1016/j.clinbiochem.2018.05.010 2. Emmens JE et al. (2019): Proenkephalin, an opoid system surrogate marker, as a novel comprehensive renal marker in heart failure, Circ. Haeart Fail., doi: 10.1161/CIRCHEARTFAILURE.118.005544 3. Gayat E. et al, (2018) Back-to-back comparison of penKID with NephroCheck® to predict acute kidney injury at admission in intensive care unit, Critical Care, doi: 10.1186/s13054-018-1945-9 4. Caironi P. et al. (2018): Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock, Clin.Chem., doi 10.1373/clinchem.2018.288068 5. Rosenqvist, M. (2019): Proenkephalin A 119–159 (penKid) – a novel biomarker for acute kidney injury in sepsis: an observational study, doi: 10.1186/s12873-019-0283-9 6. Ponce, D. (2016): Acute kidney injury: risk factors and management challenges in developing countries, Int J Nephrol Renovasc Dis., doi: 10.2147/IJNRD.S104209 About sphingotec SphingoTec GmbH ("sphingotec"; Hennigsdorf near Berlin, Germany) develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary biomarkers for the diagnosis, prediction and monitoring of acute medical conditions, such as sepsis, acute heart failure, circulatory shock, and acute kidney injury in order to support patient management and provide guidance for treatment strategies. sphingotec's proprietary biomarker portfolio includes bioactive adrenomedullin (bio-ADM®), a unique biomarker for real-time assessment of endothelial function in conditions like sepsis or congestive heart failure, Proenkephalin (penKid®), a unique biomarker for real-time assessment of kidney function, and Dipeptidyl Peptidase 3 (DPP3), a unique biomarker for cardio-renal pathway disruptions leading to acute organ dysfunction. In addition, sphingotec develops a portfolio of novel biomarkers, which predict the risks of developing obesity, breast cancer and cardiovascular diseases. IVD tests for sphingotec’s proprietary biomarkers are made available as sphingotest® microtiterplate tests as well as point-of-care tests on the Nexus IB10 immunoassay platform by sphingotec’s subsidiary Nexus Dx Inc. (San Diego, CA, USA) alongside a broad menu of IB10 tests for established biomarkers for acute and critical care. About Nexus Dx Inc. and the IB10 Platform Nexus Dx Inc., a wholly-owned subsidiary of sphingotec, headquartered in San Diego, CA, USA, is a global provider of a near patient testing system and advanced diagnostic solution. The company is improving patient care by providing the medical community with rapid and reliable information at the point of care (POC), delivering patient information when and where it is needed most. The company has invested over $160m to develop and market the IB10 analyzer system which, without the need for sample preparation, automatically separates plasma from whole blood with subsequent reliable and quantitative detection of biomarkers in the plasma by means of antibodies. With a hands-on-time of less than 3 minutes the easy-to-use system provides in only 20 minutes test results for biomarkers that are crucial in the management of critical care patients. The portfolio of IB10 assays includes tests for established critical care parameters such as Procalcitonin, Troponin I, CK-MB, Myoglobin, NT-proBNP, and D-Dimer as well as tests for sphingotec’s proprietary biomarkers such as DPP3, an assay for Dipeptidyl Peptidase 3, a unique and proprietary biomarker for cardio-renal pathway disruptions leading to acute organ dysfunction, and Proenkephalin (penKid®), a unique and proprietary biomarker for real-time assessment of kidney function. An IB10 assay for bioactive Adrenomedullin (bio-ADM®), a unique and proprietary biomarker for endothelial function is expected to be launched by mid-2020. About penKid® sphingotec’s proprietary biomarker Proenkephalin (penKid®) is a functional kidney biomarker that works in whole blood, is independent from comorbidities and inflammation and provides timely information about changes in kidney function. penKid® is a surrogate marker for the gold standard of glomerular filtration rate (GFR) and indicates two days earlier than the standard-of-care the development of acute kidney injury (AKI) in critically ill patients. These features enable physicians to predict, diagnose, and closely monitor worsening and improving kidney function in emergency departments and intensive care units.
